Hernia Repair Devices Market Size, Growth, Trends, Demand & Application 2027
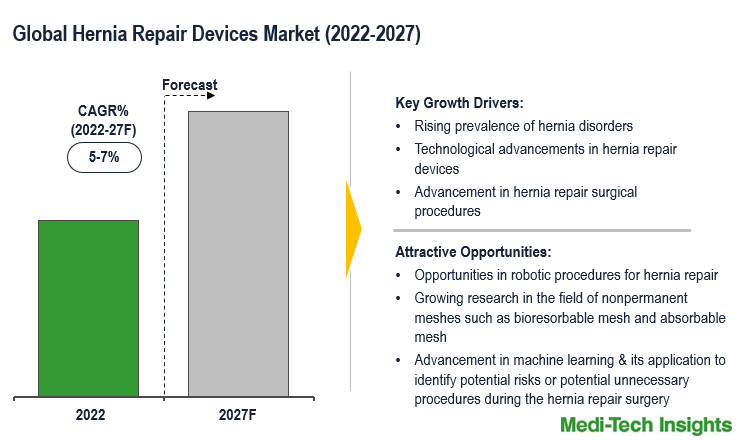
The Global Hernia Repair Devices Market is expected to grow at a decent rate of ~5-7% by 2027. Growing cases of hernia disorders, technological advancements in hernia repair devices, the growing adoption of mesh in hernia repair surgeries, rising geriatric populations, and the high adoption of hernia repair surgical procedures are some of the key factors driving the market growth.
A hernia is a sac formed by the lining of the abdominal cavity (peritoneum). The sac comes through a hole or weak area in the strong layer of the belly wall that surrounds the muscle. This layer is called the fascia. Many types of hernia have been described but the most common ones are inguinal (groin), femoral (thigh), umbilical, hiatal, and incisional hernias. Hernia repair devices provide permanent reinforcement to the repaired hernia and support damaged tissue around it as it heals.
Growing Adoption of Hernia Mesh Products for Hernia Repair Fuels the Demand of
Hernia mesh is a biomaterials-based implant class that has been successfully translated to clinical applications. They are generally divided into two categories: synthetic mesh made of synthetic materials such as polypropylene, polyethylene terephthalate, and polytetrafluoroethylene. The other category is biologic/biological mesh made of animal-derived or allogeneic materials. The use of hernia mesh products for surgical repair or reconstruction of anatomical defects has been widely adopted. The hernia mesh firmly reinforces the weakened area and provides tension-free repair that facilitates the incorporation of fibro collagenous tissue. According to the FDA, hernia mesh is used in about 90% of hernia repair surgeries.
For instance,
- In March 2023, TELA Bio, Inc announced that they have received the U.S. Food and Drug Administration 510(k) clearance for the Company's OviTex PRS Long-Term Resorbable product.
- In April 2022 – Ariste Medical announced they have received the United States Food and Medication Administration permission to market its synthetic hernia mesh with drug embedding in the country under the 510(k) exemption.
Robotic Surgeries Opens Up Growth Opportunities in the Hernia Repair Devices Market
Hernia repair is one of the most common surgical procedures performed globally. About 20 million patients worldwide suffer abdominal hernias every single year, a common condition that can only be addressed through surgical intervention. The number of procedures has been increasing and it is expected to rise further due to several risk factors such as obesity and prior abdominal surgeries. Robotic surgery has emerged as a newer technique for treating smaller hernias or weak areas, and also to reconstruct the abdominal wall. One of the biggest differences between laparoscopic surgery and robotic surgery is that the use of the robot provides excellent three-dimensional images of the inside of the abdomen (vs. the two-dimensional images of laparoscopic surgery). Robotic surgery also allows the surgeon to easily use stitches to sew tissue and meshes inside the abdomen. Advantages of robotic surgery include three-dimensional images of the inside of the abdomen, smaller scars, and less pain. The benefits associated with robotic surgeries are expected to boost the demand for hernia repair devices in the coming years.
Growing Prevalence of Inguinal Hernia Drives the Growth of the Global Hernia Repair Devices Market
Inguinal hernias account for 75% of all abdominal wall hernias, with a lifetime risk of 27-43% in men and 3-6% in women. The lifetime risk to develop an inguinal hernia is 27–43% for men and 3–6% for women. Despite all advances, ~11% of all patients suffer from a recurrence and 10–12% from chronic pain following primary inguinal hernia repair. The risk factors for inguinal hernia (IH) include family history, previous contra-lateral hernia, male gender, age, abnormal collagen metabolism, prostatectomy, and low body mass index. The rising burden of inguinal cases among the growing population and aging society is likely to increase the demand for hernia repair devices.
Key Market Constraints/Challenges: Hernia Repair Devices Market
The high costs of the hernia repair procedures and the risk & complications associated with hernia repairs such as pain, infection, hernia recurrence, adhesion, and bowel obstruction is likely to hamper the growth of the global hernia repair devices market in the coming years. Also, in some cases, patients report mesh failure and migration after hernia mesh surgery.
North America is Expected to Hold a Larger Share in the Hernia Repair Devices Market
From a geographical perspective, North America holds a larger market share of the hernia repair devices market. This can be mainly attributed to the growing prevalence of hernia disorders, higher adoption of new and advanced hernia repair devices, advancements in hernia repair surgery technology, favorable reimbursement policies in key markets, and growing demand for hernia repair procedures in the region. However, the Asia-Pacific region is expected to witness strong growth in the coming years due to an aging population, and growing innovations in hernia repair devices market in the region.
Competitive Landscape Analysis: Hernia Repair Devices Market
Some of the key and well-established players operating in the global hernia repair devices market are listed below:-
- Medtronic
- B Braun Melsungen AG
- Cook Medical
- W.L. Gore & Associates
- Ethicon Inc.
- C.R Bard Inc.
- Atrium
- LifeCell Corporation
- Baxter International Inc.
- TELA Bio
- AbbVie
- Integra
- Herniamesh S.R.L.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Hernia Repair Devices Market
Leading players operating in the global hernia repair devices market are adopting both organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner a larger market share.
For instance,
- In February 2023, TELA Bio, Inc announced the launch of two additional configurations of its OviTex LPR device. The new configurations are 15 x 20 cm and 15 x 25 cm ellipses designed for ventral and incisional hernias.
- In December 2022, Deep Blue Medical Advances announced that they have received an additional 510(k) clearance from the United States FDA for its T-Line Hernia Mesh for the subway technique in open hernia surgery.
The global hernia repair devices market is a growing market and is expected to gain further momentum in the upcoming years due to a strong emphasis on developing new minimally invasive procedures, investment in R&D to introduce several advanced products, technological advancements in hernia repair devices, and aggressive organic and inorganic growth strategies followed by the market players in hernia repair devices market.
Key Strategic Questions Addressed in this Research Report:-
- What is the market size & forecast for the hernia repair devices market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the hernia repair devices market?
- How has Covid-19 impacted the hernia repair devices market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the hernia repair devices market?
- What is the investment landscape of hernia repair devices market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the hernia repair devices market?
- What are the key strategies adopted by players in hernia repair devices market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2.Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunities
3.2. Industry Speaks
3.3. Key Market Trends
4. COVID-19 Impact on Hernia Repair Devices Market
5. Global Snapshot: Hernia Repair Procedures
6. Global Hernia Repair Devices Market- Size & Forecast (2019-2027), By Product
6.1. Hernia Mesh
6.1.1. Biological Mesh
6.1.2. Synthetic Mesh
6.2. Hernia Fixation Devices
6.2.1. Sutures
6.2.2. Tack Applicators
6.2.3. Glue Applicators
7. Global Hernia Repair Devices Market- Size & Forecast (2019-2027), By Procedure Type
7.1. Open/Conventional Procedures
7.2. Laparoscopic Procedures
7.3. Robotic Procedures
8. Global Hernia Repair Devices Market- Size & Forecast (2019-2027), By Region
8.1. North America (U.S. & Canada)
8.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
8.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
8.4. Rest of the World (Latin America, Middle East & Africa)
9. Competitive Landscape
9.1. Key Players and their Competitive Positioning
9.1.1. Market Share Analysis (2022)
9.1.2. Segment-wise Player Mapping
9.2. Key Strategies Assessment, By Player (2020-2022)
9.2.1. New Product & Service Launches
9.2.2. Partnerships, Agreements, & Collaborations
9.2.3. Mergers & Acquisitions
9.2.4. Geographic Expansion
10. Key Companies Scanned (Indicative List)
10.1. Medtronic
10.2. B Braun Melsungen AG
10.3. Cook Medical
10.4. W.L. Gore & Associates
10.5. Ethicon Inc.
10.6. C.R Bard Inc.
10.7. Atrium
10.8. LifeCell Corporation
10.9. Baxter International Inc.
10.10. TELA Bio
10.11. Herniamesh S.R.L.
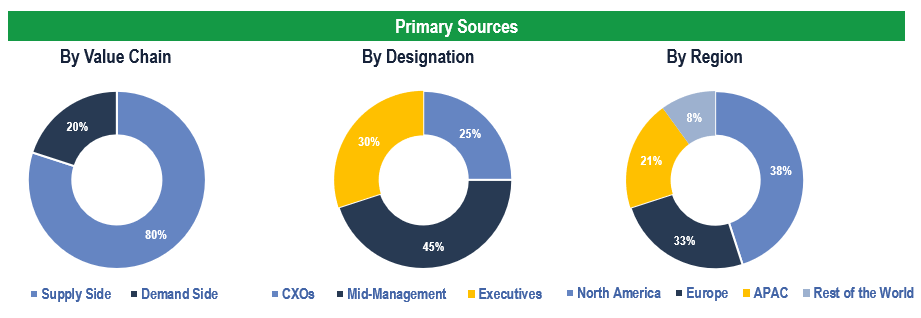
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Clinics, Ambulatory Surgical Centers, and Other End Users.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.