Electronic Trial Master File (eTMF) Systems Market – Revolutionizing Clinical Trials With Success
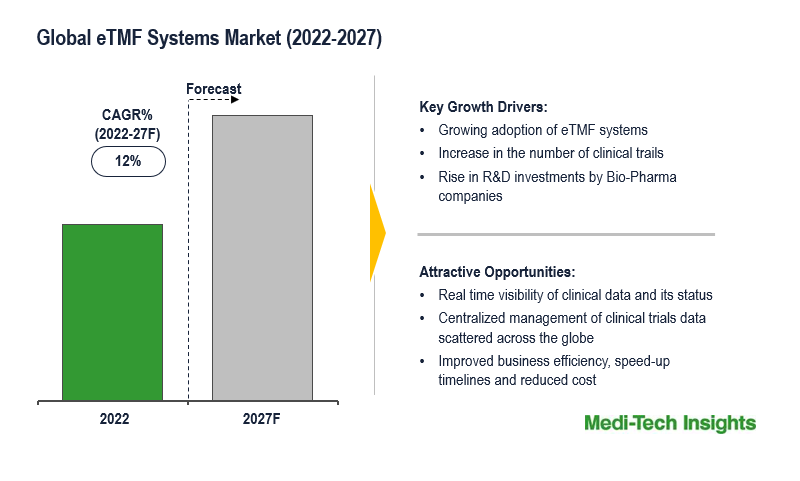
The Global Electronic Trial Master File (eTMF) Systems market is expected to witness a growth rate of 12% by 2027. The growth in the number of clinical trials, a surge in the volume of data generated, and an increase in the number of eTMF system users are some of the key factors driving the eTMF systems market growth. However, inadequate trained professionals and clinical data privacy are some of the challenges that are likely to hamper the global growth of the eTMF systems market.
An electronic Trial Master File (eTMF) is a content management system for the Bio-Pharma industry which includes protocols, strategies, and tools used throughout the lifecycle of the clinical trial. An eTMF digitally captures, shares, and stores the essential documents and content generated in a clinical trial.
Multiple Benefits Offered by eTMF Systems Drive the Electronic Trial Master File Systems Market
Post Covid-19 pandemic, the number of clinical trials and volume of data generated increased exponentially, which in turn has fueled the adoption of eTMF systems by Bio-Pharma companies. The eTMF systems give real-time visibility of the clinical trial data and activities conducted at scattered sites across the world. The benefits of adopting eTMF are increased business efficiency, speedup timelines, reduced cost and risk.
For instance,
- In June 2022, Anju Software Inc., announced the introduction of its new cloud-based eTMF product. The product will expedite the collaboration between CRO’s, sponsor and sites to manage the clinical data efficiently in a regulatory-compliant environment.
Resurgence of eTMF Systems Market Post Co-vid 19 Pandemic
The Covid-19 Pandemic has triggered a change in execution of the clinical trials. Due to travel bans and lockdowns, virtual clinical trials were initiated by Bio-Pharma companies by adopting remote patient monitoring, telehealth, wearable devices and apps to ensure the patient safety. The vast number of clinical trials conducted for rapid development of vaccines during the pandemic had also burdened the healthcare sector. With growth in the number of trials and its data, there was a shift from manual TMF systems to eTMF systems, for seamless management of data in decentralized clinical trials. The other factor which had set in motion this shift was the clinical data retention regulation policy to maintain the data for 25 years by the Sponsors/CRO’s.
For instance, to comply with the eTMF retention regulation, Arkvium Ltd. a software and services company provide eTMF digital archiving and preservation solution to ensure that data is securely stored for long term mandated by the regulators.
Key Constraints/ Challenges: eTMF Systems Market
The dearth of trained professional in operating eTMF systems, clinical data privacy concerns, and cyber-attacks are some of the key challenges that are likely to hamper the growth of the eTMF systems market in the upcoming years.
North America is Expected to Continue to Hold a Major Share in the eTMF Systems Market
From a geographical perspective, North America is expected to hold a major market share of the eTMF systems market. This can be mainly attributed to the growing number of clinical trials & volume of data generated, favorable funding environment to support clinical trials, well-developed healthcare IT infrastructure, and presence of several key players in this region However, the Asia-Pacific region is expected to witness a strong growth in the electronic trial master file systems market in the upcoming years due to flourishing Biopharma industry, growing outsourcing of clinical trials in the region, and rapid development of vaccines by Biopharma industry due to Covid-19 Pandemic.
Competitive Landscape Analysis: eTMF Systems Market
Some of the established and leading players operating in the eTMF Systems market are listed below:-
- Veeva Systems
- Aris Global LLC
- Master Control Inc
- Montrium
- Clinevo Technology
- Oracle Corporation
- Phlexglobal
- Transperfect
- Aurea Software
- Labcorp (Covance Inc.)
- Sureclinical
Organic and Inorganic Growth Strategies Adopted by Leading Market Players to Establish Their Foothold in the Global eTMF Systems Market
The key players operating in the eTMF Systems market are adopting both organic and inorganic growth strategies such as launching new products/technologies, collaborations, and acquisitions to garner higher market share.
For instance,
- In February 2023, TransPerfect Life Sciences announced that Surrozen has selected its Trial Interactive eClinical platform, including electronic Trial Master File (eTMF) and TMF solutions to create a centralized, quality-focused approach to TMF management.
- In March 2022, Montrium announced that Resonance Health has chosen eTMF Connect for its Clinical Trial Management Solution.
The eTMF systems market is a growing market and is expected to gain further momentum in the upcoming years with growing adoption of the eTMF systems, rising R&D investments by pharma-biotech companies, favorable government funding & grants to support clinical trials, and aggressive organic and inorganic growth strategies followed by leading market players.
Key Strategic Questions Addressed in this Research Report:
- What is the market size & forecast for the eTMF systems market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the eTMF systems market?
- How has Covid-19 impacted the eTMF systems market?
- What are the major growth drivers, restraints/challenges impacting the electronic trial master file systems market?
- What are the opportunities prevailing in the eTMF systems market?
- What is the investment landscape of eTMF systems market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the eTMF systems market? What is the competitive positioning of key players?
- Who are the new players entering the eTMF systems market?
- What are the key strategies adopted by leading players in eTMF systems market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2. Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunities
3.1.4. Market Trend
3.2. Industry Speaks
3.3. Technological Advancements
4. Snapshot: Global Clinical Trials Landscape
5. Global Electronic Trial Master File (eTMF) System Market - Size & Forecast (2019-2027), By Component
5.1. Services
5.2. Software
6. Global Electronic Trial Master File (eTMF) System Market - Size & Forecast (2019-2027), By Delivery Mode
6.1. Cloud-based eTMF
6.2. On-Premise eTMF
7. Global Electronic Trial Master File (eTMF) System Market - Size & Forecast (2019-2027), By End-User
7.1. Pharmaceutical & Biotechnology Companies
7.2. Contract Research Organizations (CRO’s)
7.3. Other End-users
8. Global Electronic Trial Master File (eTMF) System Market - Size & Forecast (2019-2027), By Region
8.1. North America (U.S. & Canada)
8.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
8.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
8.4. Rest of the World (Latin America, Middle East & Africa)
9. Competitive Landscape
9.1. Key Players and their Competitive Positioning
9.1.1. Key Players & Their Competitive Positioning (2022)
9.1.2. Segment-wise Player Mapping
9.2. Key Strategies Assessment, By Player (2020-2022)
9.2.1. New Product & Service Launches
9.2.2. Partnerships, Agreements, & Collaborations
9.2.3. Mergers & Acquisitions
9.2.4. Geographic Expansion
10. Key Companies Scanned (Indicative List)
10.1. Aris Global LLC
10.2. Master Control, Inc.
10.3. Montrium, Inc.
10.4. Clinevo Technology
10.5. Oracle Corporation
10.6. Phlexglobal
10.7. Transperfect
10.8. Aurea Software
10.9. Other Key Players
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
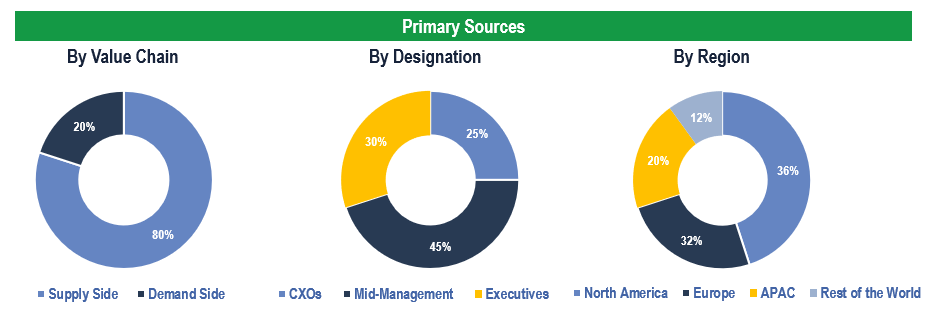
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical/Biotechnology Companies, CROs and Other End Users.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.