lipid-nanoparticle-cdmo-market-size-share-growth-trends-and-competitive-analysis-report-2027
Lipid nanoparticles are nanoparticles composed of lipids. These are spherical vesicles made of ionizable lipids, and lipid nanoparticles that a pivotal role in effectively protecting and transporting mRNA to cells. Over the years, it has emerged as a promising vehicle for delivering a variety of therapeutics.
Lipid Nanoparticles (LNPs) Acceptance in the Fight Against COVID-19 Fuels the Growth of the Lipid Nanoparticles CDMO Market
Lipids are molecules that make up the building blocks of living cells and are extremely important for mRNA-based drugs. The mRNA is surrounded by a lipid nanoparticle (LNP) composed of specific lipids. The LNP guards the mRNA from degradation and delivers it safely into the cell, where it is released. Currently, LNPs are considered the most advanced drug delivery system and due to their versatility have gained acceptance globally in the fight against COVID-19.
“The effectiveness of mRNA vaccines against COVID-19 bolstered the demand for lipid nanoparticle (LNP)-mediated drug delivery for RNA vaccines and therapeutics. As projects entered the commercialization stage from the clinical trial stage, developers were more challenged to ensure consistency in LNP formulation as the necessary quantities increased.”-Senior Scientist, Lipid Nanoparticle CDMO, U.S.A
Strong Pipeline of Lipid Nanoparticles (LNP)-Related Drugs Spurs the Demand of Lipid Nanoparticles CDMO Market
LNPs possess the capability to effectively deliver unstable genetic materials such as mRNA and gene therapies into cells while protecting them. Globally, more than 2,000 LNP-related pipeline drugs are in the development stage. The demand for contract manufacturing for manufacturing drugs for clinical trials and commercial mass production is expected to rise manifolds in the upcoming years. Citing the growth potential of LNPs, all leading market players are also increasingly adopting inorganic growth strategies to venture into the global lipid nanoparticles CDMO market.
For instance, in May 2023, Inventage Lab signed a co-development and manufacturing (CDMO) commercialization agreement with EuBiologics for LNP production
Growing Investments by Private Equity Companies in Lipid Nanoparticle CDMOs Drives the Lipid Nanoparticles CDMO Market Growth
Citing the lucrative growth prospects of the global lipid nanoparticles CDMO market, several private equity companies have also invested in Lipid Nanoparticle CDMOs.
- For instance, in June 2023, Vernal Biosciences, an mRNA and LNP Contract CDMO announced the completion of a $20MM funding round, led by Ampersand Capital Partners and Charles River Labs with participation from Dynamk Capital and the Vermont Center for Emerging Technologies to expand its GMP mRNA and LNP manufacturing capabilities.
- In August 2022, Phosphorex, a drug delivery-focused CDMO providing microspheres and polymer and lipid nanoparticles, announced a majority recapitalization by Ampersand Capital Partners, a private equity firm specializing in growth equity investments in the healthcare sector.
Competitive Landscape Analysis: Lipid Nanoparticles CDMO Market
Some of the key and promising players operating in the global lipid nanoparticles CDMO market are Evonik Health Care, Merck, CordenPharma, Phosphorex, eTheRNA Manufacturing, Fujifilm Pharmaceuticals, Helix Biotech, BIOVECTRA, Vernal Biosciences, among others.
Organic and Inorganic Growth Strategies Adopted by the Market Players to Establish Their Foothold in the Global Lipid Nanoparticles CDMO Market
Key players operating in the worldwide lipid nanoparticles CDMO market are adopting both organic and inorganic growth strategies such as expanding manufacturing capabilities, acquiring related firms, and entering into agreements and collaborations to garner a higher market share.
For instance,
- In March 2023, Evonik open new facilities in Lafayette, Indiana, in the United States and Hanau, Germany for pharmaceutical lipids.
- In March 2022, CordenPharma increased its lipid manufacturing capacity to support mRNA vaccines.
- In January 2022, Merck signed a definitive agreement to acquire Exelead, a full-service CDMO, focusing on Lipid Nanoparticle formulations. The acquisition enhances Merck’s mRNA and lipid manufacturing capabilities.
- In December 2021, WACKER and CordenPharma signed a development partnership in the field of Lipid Nanoparticle formulation. The two companies planned to jointly develop know-how and processes for the manufacturing of Lipid Nanoparticles (LNPs) to be used to meet the growing market demand.
The global lipid nanoparticles CDMO market is expected to gain a consistent momentum in the upcoming years due to the growing application horizon of lipid nanoparticles across vaccines, cancer treatments, gene therapy products, and aggressive organic and inorganic growth strategies adopted by the global market players.
Key Strategic Questions Addressed in this Research Report are as follows:-
- What is the market size and forecast for the Lipid Nanoparticles CDMO Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Lipid Nanoparticles CDMO Market?
- What are the major growth drivers, restraints/challenges impacting the Global Lipid Nanoparticles CDMO Market?
- What are the opportunities prevailing in the Lipid Nanoparticles CDMO Market?
- What is the investment landscape of the Global Lipid Nanoparticles CDMO Market?
- Which region has the highest share in the market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players in Lipid Nanoparticles CDMO Market?
- Who are the new players entering the Lipid Nanoparticles CDMO Market?
- What are the key strategies adopted by players working in the Lipid Nanoparticles CDMO Market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2. Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunities
3.2. Industry Speaks
4. Lipid Nanoparticles CDMO Market - Size & Forecast (2019-2027), By Services
4.1. Discovery Research (Formulation Development)
4.2. Preclinical Development (GLP Batch Manufacturing)
4.3. Clinical Trials (CTM Manufacturing)
4.4. Commercial Launch (Product Manufacturing)
5. Lipid Nanoparticles CDMO Market - Size & Forecast (2019-2027), By End-user
5.1. Pharmaceutical Companies
5.2. Biotech Companies
5.3. Other End-users
6. Lipid Nanoparticles CDMO Market - Size & Forecast (2019-2027), By Region
6.1. North America (U.S. & Canada)
6.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
6.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
6.4. Rest of the World (Latin America, Middle East & Africa)
7. Competitive Landscape
7.1. Key Players and their Competitive Positioning
7.1.1. Competitive Positioning of Key Players (2022)
7.1.2. Service Offerings Assessment, By Players
7.2. Key Strategies Assessment, By Player (2021-2023)
7.2.1. New Service Launches
7.2.2. Partnerships, Agreements, & Collaborations
7.2.3. Mergers & Acquisitions
7.2.4. Other Developments
8. Key Companies Scanned (Indicative List)
8.1. Evonik Health Care
8.2. Merck
8.3. CordenPharma
8.4. Phosphorex
8.5. eTheRNA Manufacturing
8.6. Fujifilm Pharmaceuticals
8.7. BIOVECTRA
8.8. Vernal Biosciences
8.9. Helix Biotech
8.10. Other Players
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
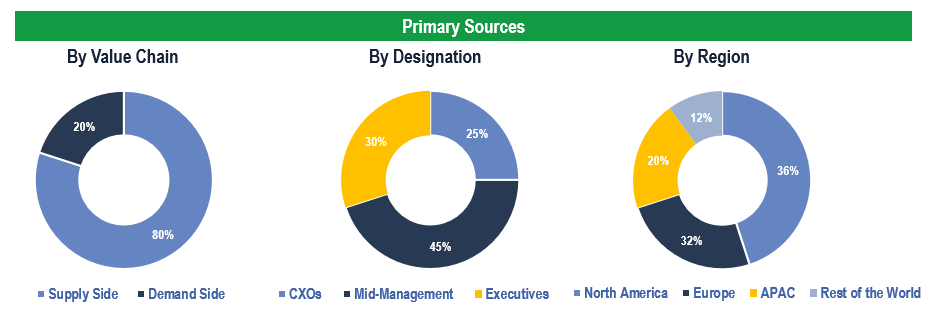
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical & Biotech Companies.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.