Global AI in Pharmacovigilance Market Size & Trends Report Segmented by Component (Software, Services), Deployment (On-premises, Cloud-based), End-user (Pharmaceutical & Biotech Companies, CROs) & Regional Forecasts to 2030
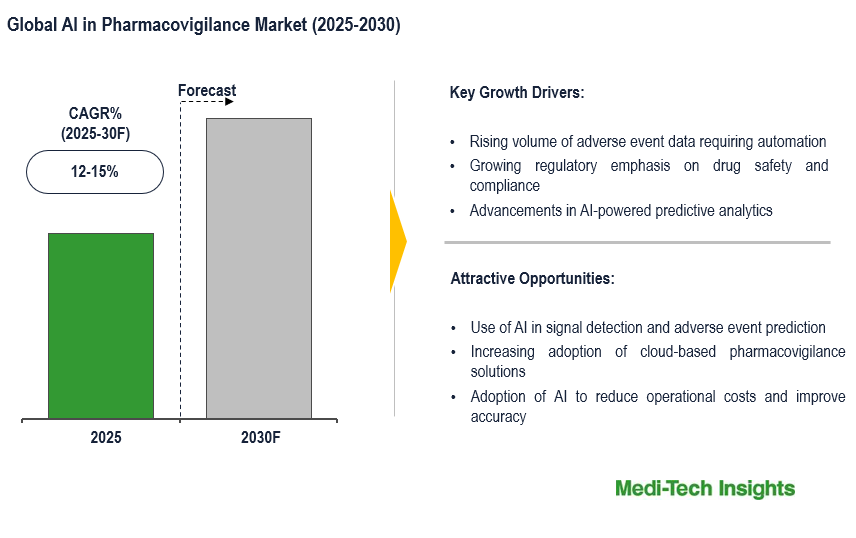
The AI in pharmacovigilance market is expected to grow at a CAGR of 12–15% from 2023 to 2030, driven by the need to automate adverse event data processing, stringent regulatory requirements, advancements in AI analytics, cost reduction benefits, and the increasing use of real-world evidence. These factors reshape pharmacovigilance processes, ensuring faster and more accurate safety assessments. However, high implementation costs and data privacy concerns pose challenges to the market growth. To learn more about the research report, download a sample report.
Report Overview
Artificial intelligence (AI) in pharmacovigilance refers to applying machine learning, natural language processing, and other AI technologies to enhance the monitoring, detection, assessment, and prevention of adverse drug reactions and related issues. It helps streamline the traditionally manual processes involved in pharmacovigilance by automating data extraction, signal detection, and case processing. AI facilitates faster and more accurate analysis of large datasets, ensuring regulatory compliance and improving drug safety. The technology is particularly valuable in managing the complexities of real-world data, including unstructured information from medical records, social media, and adverse event reports.
To learn more about this report, download the PDF brochure
Growing Volume of Adverse Event Data
The increasing volume and complexity of adverse drug reaction (ADR) data are major factors driving the adoption of AI in pharmacovigilance. With the rise of global drug usage and access to various reporting channels, pharmacovigilance teams are overwhelmed by the sheer amount of structured and unstructured data generated daily. AI technologies, such as machine learning and natural language processing, enable efficient data extraction, deduplication, and classification from disparate sources, such as electronic health records, social media, and clinical trial databases. Automating these processes reduces human errors and ensures faster identification of potential safety signals, allowing quicker interventions. This capability is especially crucial as pharmaceutical companies face stringent timelines to comply with regulatory requirements and ensure public safety. By leveraging AI, the industry can handle data growth while maintaining cost efficiency and precision.
Integration of AI with Real-World Data Analytics
One significant advancement boosting the market is the integration of AI with real-world data (RWD) analytics in pharmacovigilance. RWD, sourced from electronic medical records, wearable devices, and patient registries, offers valuable insights into drug safety and efficacy in real-life settings. AI-powered algorithms can process and analyze these vast datasets to detect adverse drug reactions and patterns that might go unnoticed in clinical trials. Additionally, AI enables the identification of rare or long-term side effects through predictive modeling and signal detection. This integration enhances proactive safety monitoring, improves decision-making, and aids in personalized medicine approaches. As regulators increasingly recognize the importance of RWD in drug safety evaluations, the use of AI-driven RWD analytics has become a transformative force in the pharmacovigilance landscape, offering faster and more accurate results to safeguard patient health.
To learn more about this report, download the PDF brochure
Competitive Landscape Analysis
The global AI in pharmacovigilance market is marked by the presence of established and emerging market players such as WNS, Accenture Plc, IQVIA Inc, Oracle, PAREXEL International Corporation, Cognizant and Aris Global among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Report Scope
| Report Metric | Details |
| Base Year Considered | 2024 |
| Historical Data | 2023 - 2024 |
| Forecast Period | 2025 – 2030 |
| Growth Rate | 12-15% |
| Market Drivers |
|
| Attractive Opportunities |
|
| Segment Scope | Component, Deployment and End-user |
| Regional Scope |
|
| Key Companies Mapped | WNS, Accenture Plc, IQVIA Inc, Oracle, PAREXEL International Corporation, Cognizant and Aris Global among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Global AI in Pharmacovigilance Market Segmentation
This report by Medi-Tech Insights provides the size of the global AI in pharmacovigilance market at the regional- and country-level from 2023 to 2030. The report further segments the market based on component, deployment and end-user.
-
Market Size & Forecast (2023-2030), By Component, USD Million
- Software
- Services
-
Market Size & Forecast (2023-2030), By Deployment, USD Million
- On-premises
- Cloud-based
-
Market Size & Forecast (2023-2030), By End-user, USD Million
- Pharmaceutical and Biotech Companies
- Contract Research Organizations (CROs)
- Others
-
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
- North America
Key Strategic Questions Addressed
- What is the market size & forecast of AI in pharmacovigilance market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of AI in pharmacovigilance market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2025-2026)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- AI in Pharmacovigilance Market Snapshot (2025-2030)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Rising volume of adverse event data requiring automation
- Growing regulatory emphasis on drug safety and compliance
- Advancements in AI-powered predictive analytics
- Increasing use of real-world evidence for safety monitoring
- Expanding global drug development and post-market surveillance
- Restraints
- High implementation and maintenance costs of AI systems
- Data privacy concerns and compliance challenges with regulatory norms
- Limited AI expertise and integration issues with legacy systems
- Opportunities
- Use of AI in signal detection and adverse event prediction
- Increasing adoption of cloud-based pharmacovigilance solutions
- Adoption of AI to reduce operational costs and improve accuracy
- Growing collaborations between AI companies and pharmaceutical firms
- Key Market Trends
- Integration of AI with real-world data analytics for proactive pharmacovigilance
- Adoption of blockchain for secure and transparent data sharing in pharmacovigilance
- Unmet Market Needs
- Industry Speaks
- Global AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Component, USD Million
- Introduction
- Software
- Services
- Global AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Deployment, USD Million
- Introduction
- On-premises
- Cloud-based
- Global AI in Pharmacovigilance Market Size & Forecast (2023-2030), By End-user, USD Million
- Introduction
- Pharmaceutical & Biotech Companies
- CROs
- Others
- Global AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Region, USD Million
- Introduction
- North America AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Country, USD Million
- US
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Canada
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- US
- Europe AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Country, USD Million
- UK
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Germany
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Italy
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Spain
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Europe
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- UK
- Asia Pacific (APAC) AI in Pharmacovigilance Market Size & Forecast (2023-2030), By Country, USD Million
- China
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Japan
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- India
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- China
- Latin America (LATAM) AI in Pharmacovigilance Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Middle East & Africa (MEA) AI in Pharmacovigilance Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Component (USD Million)
- Market Size & Forecast, By Deployment (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2024)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2024)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles*
(Business Overview, Financial Performance**, Products Offered, Recent Developments)
- WNS
- Accenture Plc
- IQVIA Inc
- Oracle
- PAREXEL International Corporation
- Cognizant
- Aris Global
- Other Prominent Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
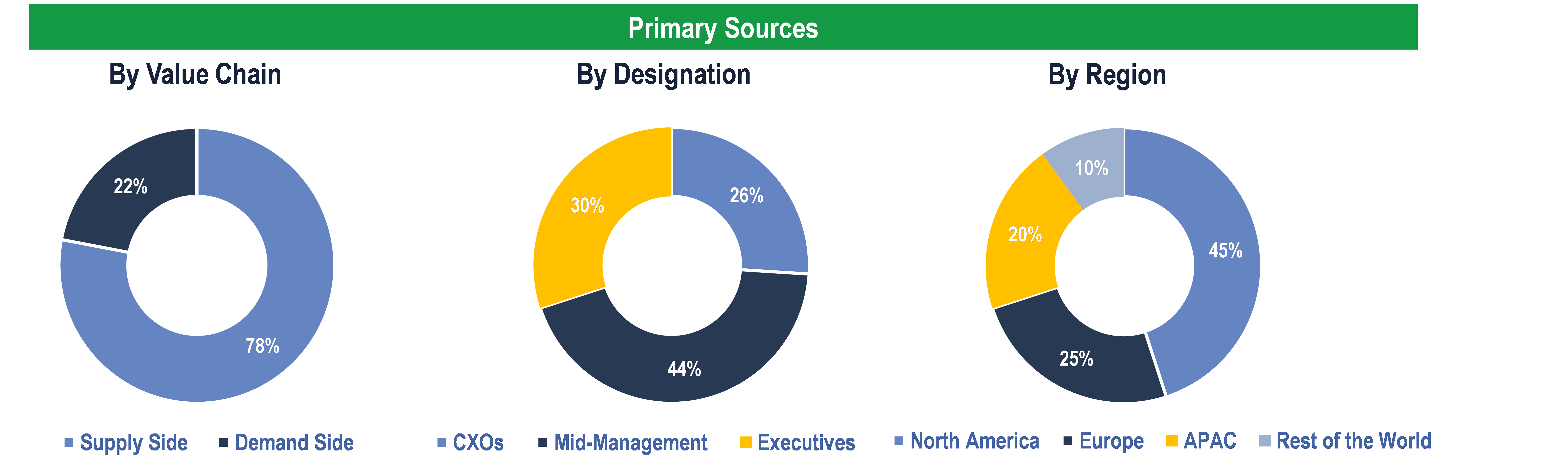
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders from Pharmaceutical & Biotech Companies, CROs and Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data