Global Prostate Cancer Diagnostics Market Size & Trends Report Segmented by Type (Adenocarcinoma, Interstitial Cell Carcinoma), Test Type (Preliminary, Confirmatory), End-user (Hospitals, Outpatient Clinics, Home-care Settings) & Regional Forecast to 2030
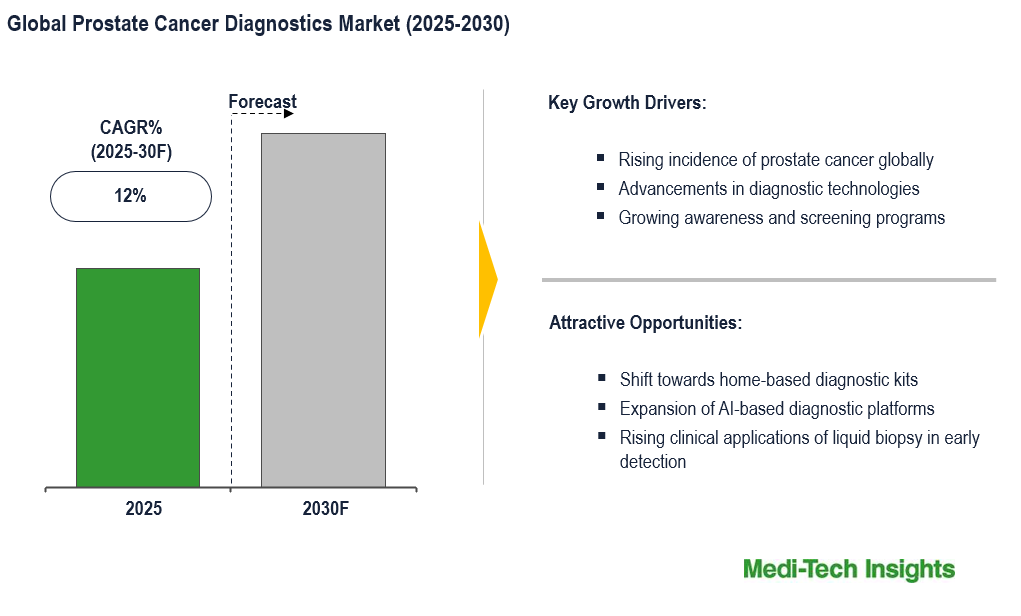
The global prostate cancer diagnostics market is expected to grow at a CAGR of ~12% during the forecast period. Rising incidence of prostate cancer, advancement in diagnostic technologies, advent of AI platforms in liquid biopsies and imaging techniques and increase in R&D investment to develop non-invasive diagnostic techniques are some of the key factors driving the market growth. However, the high cost of diagnostic tests and the need for sophisticated laboratory infrastructure are major constraints for the prostate cancer diagnostics market. To learn more about the research report, download a sample report.
Report Overview
Prostate cancer has become a common type of cancer and one of the leading causes of cancer-related deaths in men. It predominantly affects the older male population, especially those who are above 60 and are at higher risk of detection. It causes urinary problems such as blood in urine, frequent urination, and also affects bones and causes mobility issues when cancer reaches advanced stages. The disease is generally asymptomatic in its early stages, so routine screening becomes necessary, especially for high-risk individuals with a genetic predisposition or family history, as early detection can improve survival outcomes.
To learn more about this report, download the PDF brochure
Surge in cancer incidence and demand for reliable testing drives the market
According to the National Centre for Biotechnology Information (NCBI), the incidence of prostate cancer has significantly increased from 6.3 to 83.4 per 100,000 people. Aging is considered a major risk factor as it is more prevalent in men above 60. The numbers are expected to further rise in the coming years with an increase in life expectancy and a sedentary lifestyle. Further, according to an article published by WHO, in 2023, there were 1.5 million cases of prostate cancer, approximately 7.3% of total reported cancer cases, making it the most common type after lung, breast, and colorectal cancer. This rise in the number of prostate cancer cases is a primary driver of the market; thereby increasing demand for accurate and reliable diagnostics tools. For instance,
Advances in diagnostic technologies fuel market growth
Prostate-specific antigen (PSA) testing is the standard test to diagnose prostate cancer. Due to PSA’s limitations, including its inability to detect slow-growing tumours, there has been a shift toward sophisticated tools like multi-parametric MRI, liquid biopsies, and genomic assays, enabling better differentiation between indolent and aggressive tumours. These advanced techniques offer better accuracy and are more reliable, which have significantly improved early detection, therapy planning, and recovery outcomes. Some of the related developments are listed below-
- In December 2024, Siemens Healthineers acquired Advanced Accelerator Applications from Novartis, strengthening its molecular imaging capabilities with PSMA-targeted PET tracers for precision cancer diagnostics
- In October 2024, Prostate Cancer Foundation of Australia (PCFA) and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) entered into a three-year research partnership to accelerate clinical trials and drive therapeutic innovations for advanced and aggressive prostate cancer
- In May 2024, MDxHealth announced a collaboration with the University of Oxford to clinically validate its Genomic Prostate Score (GPS) test to predict prostate cancer progression following localized treatment, thereby improving risk stratification and guiding personalized patient management
- In June 2022, Roche launched the BenchMark ULTRA PLUS platform, streamlining immunohistochemistry assays to detect prostate cancer biomarkers more consistently and efficiently
Adoption of non-invasive and liquid biopsy techniques to drive demand for prostate cancer diagnostics
Traditionally, prostate cancer detection biopsy methods like tissue or transrectal ultrasound-guided (TRUS) were painful and caused infection, discomfort, and anxiety in patients. The emergence of non-invasive liquid biopsies serves as a better alternative as they detect cancer by analyzing body fluids like blood and urine. When integrated with digital PCR and next-generation sequencing (NGS), it increases the specificity and sensitivity. Advanced medical tests like MyProstateScore 2.0 and PCA3 tests urine, facilitate in early detection of cancer. For instance, some notable progress includes,
- In February 2025, Myriad Genetics entered into a strategic collaboration with PathomIQ to integrate its AI-powered PATHOMIQ_PRAD platform into Myriad’s oncology portfolio, aiming to enhance prostate cancer diagnostics by leveraging advanced image-based analytics to improve treatment decisions and support the upcoming launch of a new diagnostic test
- In September 2024, Novigenix received a €1.75 million Eurostars grant to lead the MY-SIGNATURE consortium in developing MYELO‑SCAN, the world’s first liquid biopsy designed to predict intratumoral myeloid-derived suppressor cell (MDSC) infiltration in metastatic castration-resistant prostate cancer, aiming to enhance treatment resistance prediction and improve patient outcomes
To learn more about this report, download the PDF brochure
Competitive Landscape Analysis
The global prostate cancer diagnostics market is marked by the presence of established and emerging market players such as F. Hoffmann-La Roche Ltd, Abbott, MDx Healthcare, Inc., Myriad Genetics, Siemens Healthineers, ACON Laboratories, Humasis, Opko Health Inc., Beckman Coulter, Inc., Ipsen Pharma, Diasorin, among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Report Scope
| Report Metric | Details |
| Base Year Considered | 2024 |
| Historical Data | 2023 - 2024 |
| Forecast Period | 2025 – 2030 |
| Growth Rate | ~12% |
| Market Drivers |
|
| Attractive Opportunities |
|
| Segment Scope | Type, Test Type, and End-user |
| Regional Scope |
|
| Key Companies Mapped | F. Hoffmann-La Roche Ltd, Abbott, MDx Healthcare, Inc., Myriad Genetics, Siemens Healthineers, ACON Laboratories, Humasis, Opko Health, Beckman Coulter, Inc., Diasorin, Ipsen Pharma, among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Global Prostate Cancer Diagnostics Market Segmentation
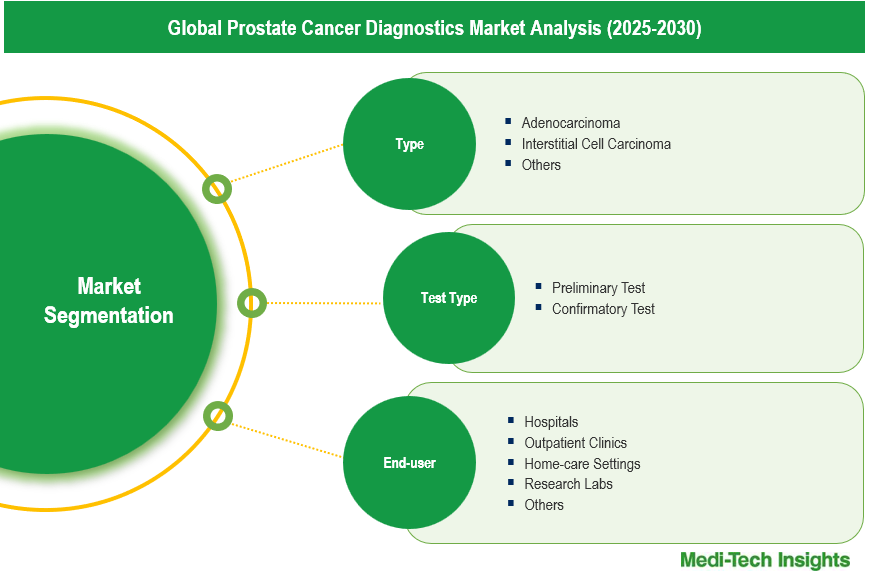
This report by Medi-Tech Insights provides the size of the global prostate cancer diagnostics market at the regional- and country-level from 2023 to 2030. The report further segments the market based on type, test type, and end-user.
Market Size & Forecast (2023-2030), By Type, USD Million
- Adenocarcinoma
- Interstitial Cell Carcinoma
- Others
Market Size & Forecast (2023-2030), By Test Type, USD Million
- Preliminary Tests
- PSA Tests
- Free PSA Test
- Total PSA Test
- Others
- Confirmatory Tests
- Pca3 Test
- Trans-Rectal Ultrasound
- Biopsy Test
- Others
Market Size & Forecast (2023-2030), By End-user, USD Million
- Hospitals
- Outpatient Clinics
- Home-care Settings
- Research Labs
- Others
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
Key Strategic Questions Addressed
- What is the market size & forecast of the prostate cancer diagnostics market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the prostate cancer diagnostics market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2025-2026)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- Prostate Cancer Diagnostics Market Snapshot (2025-2030)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Rising incidence of prostate cancer globally
- Advancements in diagnostic technologies
- Growing awareness and screening programs
- Increasing geriatric male population
- Rising R&D investments by med-tech and pharma companies
- Restraints
- High cost of diagnostic tests
- Requirement of high-end labs
- Stringent regulatory approvals
- Opportunities
- Shift towards home-based diagnostic kits
- Expansion of AI-based diagnostic platforms
- Rising clinical applications of liquid biopsy in early detection
- Expansion into emerging markets
- Key Market Trends
- Development of genomic tests for risk stratification
- Rise in demand for multi-parametric MRI
- Unmet Market Needs
- Industry Speaks
- Drivers
- Market Dynamics
- Global Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Type, USD Million
- Adenocarcinoma
- Interstitial Cell Carcinoma
- Others
- Global Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Test Type, USD Million
- Preliminary Tests
6.1.1 PSA Tests
6.1.2 Free PSA Test
6.1.3 Total PSA Test
6.1.4 Others
- Confirmatory Tests
6.2.1 Pca3 Test
6.2.2 Trans-Rectal Ultrasound
6.2.3 Biopsy Test
6.2.4 Others
- Global Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By End-user, USD Million
- Introduction
- Hospitals
- Outpatient Clinics
- Home-care Settings
- Research Labs
- Others
- Global Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Region, USD Million
- Introduction
- North America Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- US
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Canada
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- US
- Europe Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- UK
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Germany
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- France
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Italy
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Spain
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Europe
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- UK
- Asia Pacific (APAC) Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- China
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Japan
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- India
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- China
- Latin America (LATAM) Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Middle East & Africa (MEA) Prostate Cancer Diagnostics Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Type (USD Million)
- Market Size & Forecast, By Test Type (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2024)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2025)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles*
(Business Overview, Financial Performance**, Products Offered, Recent Developments)
- Hoffmann-La Roche Ltd
- Abbott Laboratories
- MDx Healthcare, Inc.
- Myriad Genetics
- Siemens Healthineers AG
- ACON Laboratories
- Humasis Co, Ltd
- Opko Health
- Beckman Coulter, Inc.
- Diasorinp. A.
- Ipsen Pharma
- Other Prominent Players
Note: *Indicative list
**For listed companies
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders from Hospitals, Med-tech companies, Research Organisations, and Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data