Autoimmune Disease Diagnosis Market Set for Rapid Growth, Size, Share, and Forecast to 2028
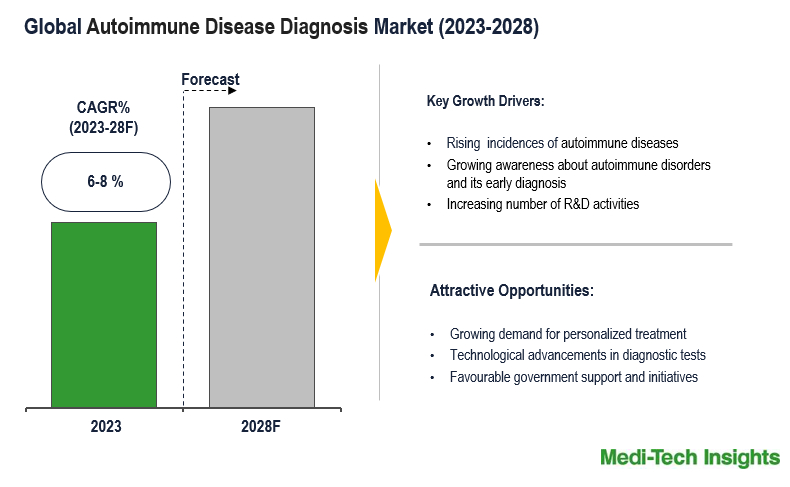
The autoimmune disease diagnosis market is projected to achieve a CAGR of 6-8 % between 2023 and 2028. This growth is propelled by factors such as the increasing incidence of autoimmune diseases, increasing awareness and growing research on autoimmune disorders, technological advancements, and the escalating demand for personalized medical solutions. To learn more about the research report, download a sample report.
Autoimmune disease diagnosis refers to identifying and confirming the presence of autoimmune disorders in individuals. This involves a comprehensive evaluation of symptoms, medical history, physical examinations, and various diagnostic tests, including blood tests, imaging studies, and tissue biopsies. The aim is to detect abnormalities in the immune system's response, such as autoantibodies or inflammation, which indicate the presence of an autoimmune condition. Prompt and accurate diagnosis is crucial for initiating appropriate treatment and management strategies to alleviate symptoms and prevent complications associated with autoimmune diseases. Autoimmune Disease Diagnosis is broadly classified into the following types:
- Serological Tests: These tests detect specific antibodies or proteins in the blood, aiding in the identification of autoimmune diseases such as rheumatoid arthritis and lupus
- In Vivo Imaging: Techniques like magnetic resonance imaging (MRI) and positron emission tomography (PET) are utilized to visualize and assess organ damage caused by autoimmune disorders
- Biopsy: Tissue samples are taken from affected organs to examine cellular changes, providing valuable diagnostic information for diseases like autoimmune hepatitis and celiac disease
- Urinalysis: Analysis of urine can reveal abnormalities indicative of certain autoimmune conditions, such as lupus nephritis
- Genetic Testing: Identifying specific genetic markers associated with autoimmune diseases aids in early diagnosis and personalized treatment strategies.
Rising Prevalence and Awareness: Shaping the Autoimmune Disease Diagnosis Market
The autoimmune disease diagnosis market is experiencing significant growth, influenced by several key factors and trends. One major driver is the increasing prevalence of autoimmune diseases globally, including conditions like rheumatoid arthritis, multiple sclerosis, and lupus. This rise in incidence contributes to a growing demand for advanced diagnostic tests to facilitate early detection and effective management of these complex conditions. Another crucial factor propelling the market forward is the continuous advancements in diagnostic technologies. Innovations in biomarker identification, genetic testing, and imaging modalities enhance the accuracy and efficiency of autoimmune disease diagnostics. These technological improvements enable healthcare professionals to detect autoimmune diseases at earlier stages, leading to more effective treatment strategies and improved patient outcomes. The increasing awareness of autoimmune diseases among patients and healthcare providers significantly drives market demand by highlighting the importance of early detection and prompting individuals to seek diagnostic tests promptly. Additionally, the rise in healthcare expenditure, particularly in developed regions, facilitates broader access to advanced diagnostic technologies, further contributing to the overall growth of the autoimmune disease diagnosis market. The convergence of these factors underscores the dynamic and expanding nature of the market as it addresses the challenges posed by autoimmune diseases on a global scale. For instance,
- In June 2022, the FDA cleared Thermo Scientific EliA RNA Pol III and EliA Rib-P tests, expanding the diagnostic capabilities for Systemic Sclerosis (SSc; scleroderma) and Systemic Lupus Erythematosus (SLE). These additions enhance the EliA portfolio, offering a comprehensive range of automated connective tissue disease tests for clinicians
- In May 2022, Thermo Fisher Scientific launched the Phadia 2500+ series of instruments in the U.S., designed for autoimmune testing. These high-capacity instruments offer intuitive operation and enable instrument consolidation, providing additional automation for EliA autoimmune diagnostics and other autoimmune diseases, including Connective Tissue Disease (CTD), Rheumatoid Arthritis, and Autoimmune Thyroid Disease among others
To learn more about this report, download the PDF brochure
Tailored Solutions: Advancing Autoimmune Disease Diagnosis with Personalized Medicine and POCT
The shift towards personalized medicine is significantly shaping the landscape of autoimmune disease diagnosis. This trend is propelled by advancements in molecular diagnostics and the integration of genomic data, which enable tailored treatment strategies based on individual patient profiles. By leveraging these innovative approaches, healthcare providers can offer more precise and effective interventions, driving the demand for personalized diagnostic tests in the autoimmune disease sector. Additionally, there is an increasing adoption of point-of-care testing (POCT) in the realm of autoimmune disease diagnostics. The preference for POCT devices stems from their ability to provide rapid results and convenience, allowing for timely diagnosis and management of autoimmune conditions. As a result, POCT devices are gaining traction across various healthcare settings, contributing to the evolving landscape of autoimmune disease diagnosis market.
Autoimmune Disease Diagnosis Market: Key Constraints/Challenges
The autoimmune disease diagnosis market faces several challenges hindering its efficiency and growth. These include the complexity and heterogeneity of autoimmune diseases, which often present with overlapping symptoms and require specialized diagnostic approaches. Additionally, limited awareness and understanding among healthcare professionals about autoimmune conditions contribute to delayed or missed diagnoses. The lack of standardized diagnostic criteria and biomarkers poses challenges in accurately identifying and monitoring autoimmune diseases, leading to variability in diagnostic practices and outcomes. Furthermore, the high cost associated with autoimmune disease diagnostic tests poses an additional challenge, limiting access for patients and healthcare facilities, particularly in resource-constrained settings.
Regional Segmentation of the Autoimmune Disease Diagnosis Market
North America dominates the autoimmune disease diagnosis market due to factors such as well-established healthcare infrastructure, high prevalence of autoimmune diseases, and technological advancements in diagnostic techniques. The region's robust research and development activities, coupled with favorable reimbursement policies, further contribute to market growth. Europe holds a significant share in the autoimmune disease diagnosis market, driven by factors like increasing awareness about autoimmune diseases, rising healthcare expenditure, and government initiatives to improve healthcare infrastructure.
The Asia Pacific region is experiencing rapid growth in the autoimmune disease diagnosis market, attributed to factors such as the increasing prevalence of autoimmune diseases, improving healthcare infrastructure, and rising healthcare expenditure. Emerging economies like China, India, and Japan are witnessing a surge in demand for diagnostic tests due to the growing awareness about autoimmune diseases and improving access to healthcare services. Additionally, the presence of untapped market opportunities and the adoption of advanced diagnostic technologies contribute to the region's market growth potential.
Autoimmune Disease Diagnosis Market: Competitive Landscape
Some of the key players operating in the market are Abbott Laboratories, Biomérieux, Trinity Biotech, Bio-rad Laboratories, Thermo Fisher Scientific, Danaher Corporation, Quest Diagnostics INC., HYCOR Biomedical, Siemens Healthineers and AESKU Group, among others.
Get a Sample Report for Competitive Landscape Analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In December 2023, Thermo Fisher Scientific announced an exclusive distribution agreement with Aesku. Group to market, sell and support their FDA-cleared IFA products, automated instruments, and software in the United States. This collaboration enables Thermo Fisher to offer laboratories a comprehensive suite of automated diagnostic systems and methodologies in the U.S. market, complementing its existing EliA™ autoimmune testing portfolio and Phadia™ laboratory instruments with Aesku's IFA testing and Helios® HTC systems
- In September 2023, Recipharm initiated a partnership with Ahead Therapeutics to collaborate on pioneering treatment for the rare autoimmune disease, myasthenia gravis
- In June 2023, AstraZeneca embarked on an intriguing collaboration, signing an exclusive option and license agreement with Quell Therapeutics. The partnership aims to develop several engineered T-regulator (Treg) cell therapies, which hold promise as potential cures for Type 1 Diabetes (T1D) and Inflammatory Bowel Disease (IBD) indications
The autoimmune disease diagnosis market is expected to gain momentum in the coming years due to the rising incidences of autoimmune disorders, increasing demand for drug discovery, technological advancements, and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
- What is the market size & forecast for the Global Autoimmune Disease Diagnosis Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Autoimmune Disease Diagnosis Market?
- How has COVID-19 impacted the Global Autoimmune Disease Diagnosis Market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the market?
- What is the investment landscape?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the market?
- What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Dynamics
- Global Autoimmune Disease Diagnosis Market - Size & Forecast (2021-2028), By Disease Type
- Graves Disease
- Rheumatoid Arthritis
- Thyroiditis
- Other Diseases
- Global Autoimmune Disease Diagnosis Market - Size & Forecast (2021-2028), By Test Type
- Antinuclear antibody (ANA) tests
- Routine Laboratory Tests
- Other Tests
- Global Autoimmune Disease Diagnosis Market - Size & Forecast (2021-2028), By End User
- Hospitals
- Clinical Laboratories
- Academic & Research Institutes
- Other End Users
- Global Autoimmune Disease Diagnosis Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Abbott Laboratories
- Biomérieux
- Trinity Biotech
- Bio-rad Laboratories
- Thermo Fisher Scientific
- Danaher Corporation
- Quest Diagnostics INC.
- HYCOR Biomedical
- Siemens Healthineers
- AESKU Group
- Other Players
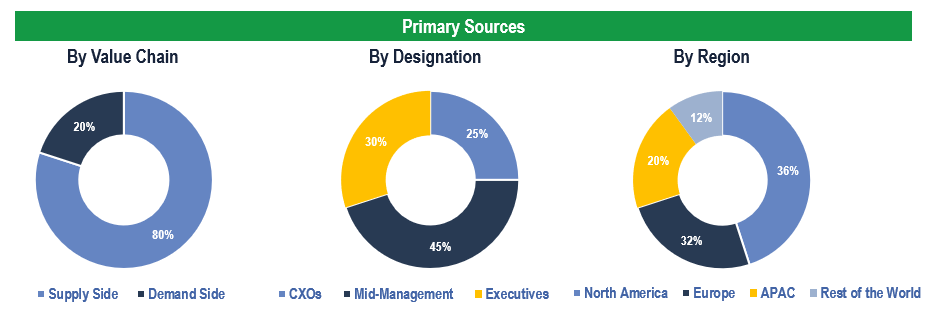
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Clinical Laboratories, and Academic & Research Institutes among others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.