Biologics Consulting Services Market Size, Share, Trends, Growth Drivers & Forecasts 2026
The global biologics consulting services market is expected to witness a CAGR of >15%, driven by the growing biopharmaceutical market, increasing R&D activities in advanced therapies like cell & gene therapy, growing biologics regulatory approvals, and increasing VC fundings for biologics.
While small molecules are either made synthetically or purified from plants, biologics are medicines made from living cells. Although small molecules comprise >90% of drugs on the market, pharma and biotech companies are gradually shifting their focus to manufacturing and developing highly profitable biologics drugs.
The Complex Nature of Biologics and its Increasing Importance Drives the Demand for Consulting Services
Compared to small molecules, biologics are large, highly complex, heterogeneous molecules, more expensive, sensitive to external conditions, need to go through challenging manufacturing processes and have stringent regulatory requirements, thus requiring a greater level of expertise.
With the increasingly complex and rapidly evolving biopharma industry, companies are looking for consultancies that are able to provide significant regulatory leadership, product development consulting, and expertise in all stages from early-stage discovery to commercialization. Moreover, the increasing importance of new biologics drugs is seen over the last few years, with biologics contributing to 25% of new molecule approvals in the US in 2020. This further boosts the demand for biologics consulting.
“Continued development of blockbuster biologics (antibody, immunosuppressants, anticancer, atopic dermatitis, etc.), a growing number of biosimilars being approved as patents continue to expire, the entrance of small, virtual pharma startups in the market with no manufacturing capacity and/or limited development expertise, and rising prevalence of chronic infectious diseases & cancer are some of the key factors that are likely to drive the global biologics consulting market.” - Head of Process Development, Leading Biologics Consulting Company, US
Rising Influx of Small Biotech Lacking Manufacturing/Development Expertise
With the increased demand for novel therapies, there is a rising influx of small biotech firms that usually lack in-house capabilities and expertise. These firms are largely reliant on biologics consulting companies for end-to-end services from the exploratory phase to commercial manufacturing. Nevertheless, big biopharma companies need to work in a complex and dynamic environment that requires strict attention to meeting new regulatory standards. They also approach consulting firms to help them to maintain a strong track record of quality, compliance, and regulatory expertise.
Mounting Demand for Advanced Technologies Consulting
Advanced therapies, such as cell and gene therapies and personalized vaccines require specialized expertise throughout the development, manufacturing, and regulatory process. Newer vaccine technologies like viral vector and mRNA where demand exceeds supply and capabilities/technical expertise may not exist even in large biopharma. Moreover, the rapidly evolving regulatory landscape makes it crucial to have up-to-date knowledge and expertise to successfully develop a product and gain a centralized market authorization (MA) in Europe and/or a Biologic License Authorization (BLA) in the US. With the rapidly growing demand for advanced biologics therapies, there is a corresponding demand for biologics consulting services.
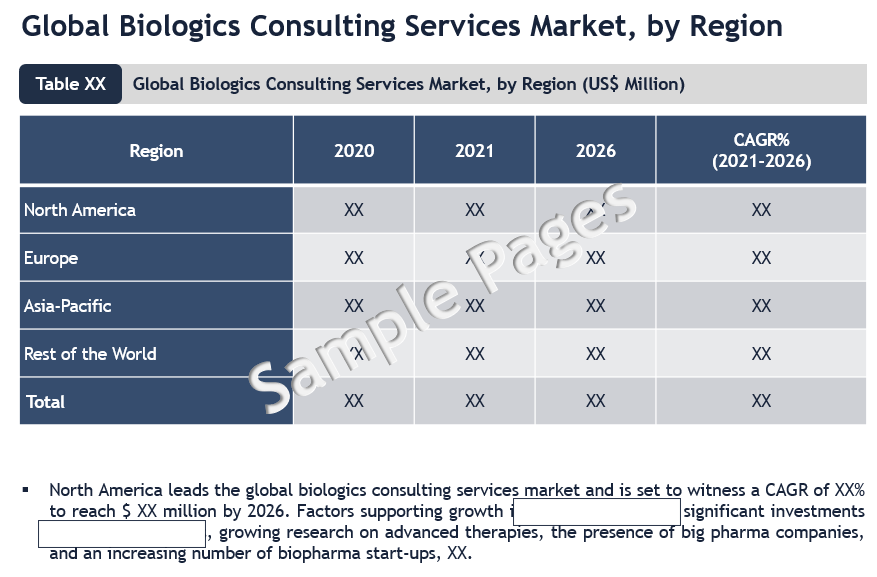
North America Leads the Adoption of Biologics Consulting Services Market
From a geographical perspective, North America has a major market share of the global biologics consulting services market. This is mainly attributed to the growing biopharmaceutical industry, favorable government policies supporting biologics innovation, the presence of biologics consulting firms, significant R&D spending in the US, strict FDA regulations, and a greater focus on advanced therapies.
Competitive Landscape Analysis: Biologics Consulting Services Market
The global biologics consulting services market is highly competitive and fragmented. Some of the key/promising players in this market include IQVIA, NNE, ProductLife Group, Clarkston Consulting, Fingerpaint, Azenta Life Sciences, Biologics Consulting Group, UDG Healthcare, ProPharma Group, APCER Life Sciences, Freyr Solutions, ADVANCED CELL & GENE THERAPY, LLC, Dark Horse Consulting, 4Clinics, eXmoor pharma concepts limited, among others.
As a key growth strategy, companies operating in the Biologics Consulting Services market are focusing on adding/expanding their portfolio with high-growth advanced therapy consulting services in key areas such as regulatory, development, manufacturing, and ultimately commercialization. Moreover, looking at the lucrative growth opportunities offered by this market, new companies are also entering this space. For instance,
- In April 2021, ProductLife Group acquired DSI which provides regulatory, technical, and consulting services for pharmaceuticals, biopharmaceuticals, and cellular and gene therapy products.
- In April 2019, Cell One Partners launched its consulting services, supporting the development of cell and gene therapies.
Key Strategic Questions Addressed
- What is the market size & forecast of the Biologics Consulting Services market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Biologics Consulting Services market?
- How has covid impacted the Biologics Consulting Services market?
- What are the major growth drivers, restraints/and challenges impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the Biologics Consulting Services market? What is the competitive positioning of key players?
- What are the key strategies adopted by players?
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
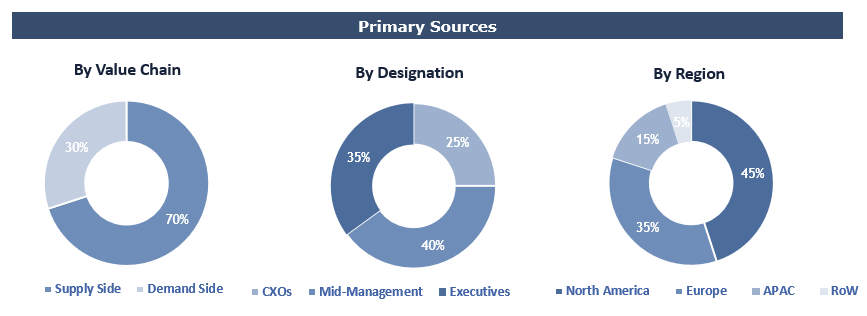
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharma, Biotech, CROs, CDMOs & Other End Users.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data.