CAR T-cell Therapy Market Size, Share, Trends, Demand, Industry Growth Analysis and Forecast 2024 – 2029
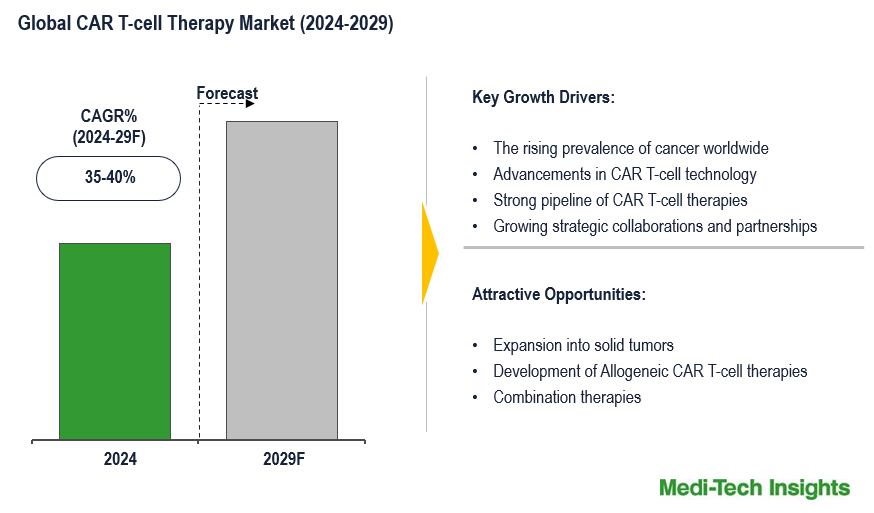
The Global CAR T-cell Therapy Market is expected to witness a growth rate of 35-40% in the next five years. The rising prevalence of cancer worldwide, advancements in CAR T-cell technology, strong pipeline of CAR T-cell therapies, growing strategic collaborations and partnerships between pharmaceutical companies, biotechnology firms, and academic institutions, increase in investments directed towards the research and development of new CAR T-cell therapies, regulatory support and approvals, growing adoption of personalized medicine, and high efficacy of CAR T-cell therapy are some of the key factors driving CAR T-cell therapy market growth. However, high cost of therapy, complex manufacturing process, potential for severe side effects, and limited availability of treatment centers are likely to hinder the market growth. To learn more about the research report, download a sample report.
Chimeric antigen receptor T-cell therapy (CAR T-cell therapy) is a type of cancer treatment that reprograms a patient's immune system to fight cancer. CAR T-cell therapy is a groundbreaking form of immunotherapy that involves modifying a patient's own T cells to better recognize and attack cancer cells. The process includes extracting T cells from the patient, genetically engineering them to express chimeric antigen receptors (CARs) that target specific cancer antigens, and then reinfusing these engineered cells back into the patient. This therapy has shown significant effectiveness in treating certain types of blood cancers, such as leukemia and lymphoma.
The Rising Prevalence of Cancer Worldwide to Drive Market Growth
The increasing prevalence of cancer worldwide is putting significant pressure on healthcare systems to effectively manage and treat a growing number of patients. According to the global world health organization (WHO) survey on universal health coverage (UHC) and cancer, in 2022, there were an estimated 20 million new cancer cases and 9.7 million deaths. Moreover, an estimated 1.24 million blood cancer cases occur annually worldwide, accounting for approximately 6% of all cancer cases. With an increasing number of cancer cases, particularly hematologic malignancies such as leukemia and lymphoma, there is a growing demand for more effective and targeted treatments. CAR T-cell therapy offers a promising solution, harnessing the body’s immune system to identify and destroy cancer cells. Traditional cancer treatments, such as chemotherapy and radiation, often have limited efficacy and severe side effects, prompting the need for innovative therapies. CAR T-cell therapy's personalized approach has demonstrated high success rates, especially in patients with relapsed or refractory cancers. As cancer incidence continues to grow, healthcare providers and patients are seeking advanced, tailored treatments like CAR T-cell therapy. This rising prevalence also attracts increased investments in CAR T-cell research and clinical trials, further propelling market growth. As more CAR T-cell therapies gain regulatory approvals and demonstrate long-term benefits, the market is poised for significant expansion in response to the global cancer burden.
“Cancer is one of the leading causes of death worldwide. The rising prevalence of cancer is fueling demand for innovative and effective therapies like CAR T-cell therapy. CAR T-cell therapy has demonstrated high success rates in clinical trials for treating certain blood cancers such as leukemia and lymphoma. The therapy has produced durable responses and shown potential to cure some patients"- Consultant Oncologist, A Leading Hospital Group, United States
Strong Pipeline of CAR T-cell Therapies to Fuel Market Growth
A robust pipeline of CAR T-cell therapies is set to fuel significant growth in the CAR T-cell therapy market. Numerous clinical trials and product developments are underway, driven by both established pharmaceutical companies and innovative biotech firms. Major players like Gilead, Novartis, Bristol Myers Squibb, and Bluebird bio are at the forefront, developing next-generation therapies targeting various cancers, including hematological malignancies and solid tumors. The approval of Kymriah and Yescarta in the US in 2017, followed by their expansion into Europe and Japan during 2018-2019, alongside growing global sales, has sparked increased investor interest in adoptive cellular immunotherapy. In recent years, the field has seen rapid advancements, with researchers working to enhance the safety and efficacy of CAR T-cell therapies through novel approaches. These developments aim to address current challenges in adoption by offering improved efficacy, safety, and more efficient delivery mechanisms, paving the way for broader clinical application. Numbers of therapies based on various CAR-T technologies being investigated in clinical trials for blood cancers, solid tumours and autoimmune diseases. For instance, as of 25 January 2023, there were 1087 CAR T-cells clinical trials on ClinicalTrials.gov (Source: NCBI). As more therapies progress through clinical trials, the market is poised for a substantial boost. According to research, the number of CAR T-cell therapy clinical trials has steadily increased with an emphasis on improving patient outcomes and tackling challenges such as antigen escape and resistance. Additionally, ongoing innovations aim to reduce treatment costs and improve manufacturing scalability, further driving market growth. With such a strong pipeline, CAR T-cell therapies are expected to become a cornerstone of cancer treatment, leading to considerable market expansion.
“The application of CAR T-cells in treating multiple myeloma holds promise because, at present, myeloma remains incurable. While various drugs are effective in achieving remission, they are unable to fully cure the disease. CAR T-cell therapy may soon be used earlier in treatment, rather than just for patients with refractory myeloma, to solidify remission and potentially lead to a cure. This shift could transform the current treatment approach by using CAR T-cells as a more proactive measure"- Haematologist-oncologist, A Leading Hospital Group, Italy
To learn more about this report, download the PDF brochure
Development of Allogeneic CAR T-cell Therapies to Offer Significant Market Opportunities
The development of allogeneic CAR T-cell therapies represents a major opportunity in the CAR T-cell therapy market. Unlike autologous CAR T-cell therapies, which require the patient's own T-cells to be harvested, modified, and reinfused, allogeneic CAR T-cell therapies use T-cells from healthy donors. This shift to donor-derived cells has the potential to streamline the production process, significantly reducing both the time and cost involved in delivering treatment. This "off-the-shelf" approach also addresses one of the major limitations of current CAR T-cell therapies i.e., the lengthy manufacturing process for autologous treatments, which can be problematic for patients in advanced stages of cancer. Allogeneic therapies can be produced in bulk and stored for immediate use, which could make them more widely accessible and available to a larger pool of patients. In addition to cost and scalability advantages, allogeneic CAR T-cell therapies are undergoing advancements to overcome immune rejection and graft-versus-host disease (GVHD), two significant challenges with donor cells. With ongoing research and clinical trials, experts believe that allogeneic CAR T-cells have the potential to transform cancer treatment, especially for patients who cannot undergo autologous therapy.
US Expected to be a Major Growth Engine in the CAR T-cell Therapy Market
The US is projected to be a major growth engine in the CAR T-cell therapy market due to several factors. The country has a strong presence of leading biopharmaceutical companies, such as Gilead and Bristol Myers Squibb, which are heavily investing in research and development to advance CAR T-cell therapies. The US FDA has also been proactive in approving CAR T-cell therapies, creating a favorable regulatory environment that accelerates market entry. Additionally, the US has a high prevalence of cancer, especially hematologic malignancies, which CAR T-cell therapies target effectively. The strong healthcare infrastructure, combined with substantial government and private funding, further supports the market’s expansion. Moreover, a growing number of clinical trials and innovations in allogeneic CAR T-cell therapies are advancing the field, ensuring more accessible and cost-effective treatments. All these factors position the US as a key driver of global market growth in this domain. The growth in APAC is driven by an increasing prevalence of cancer, rising healthcare expenditure, and expanding research and development efforts in the region. Additionally, favorable regulatory policies and the entry of international biopharmaceutical companies into APAC markets are further boosting the adoption of CAR T-cell therapies.
To learn more about this report, download the PDF brochure
Product Type Segment Analysis
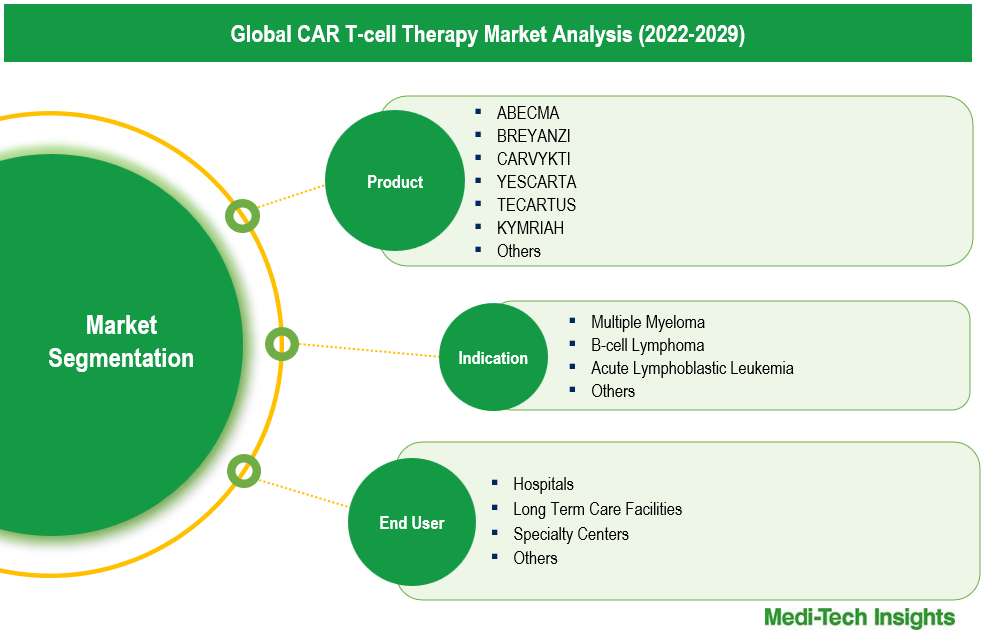
The CAR T-cell therapy market comprises of product types like: Abecma, Breyanzi, Carvykti, Yescarta, Tecartus, Kymriah, and Others. Yescarta is the largest segment. Known as Axicabtagene Ciloleucel, Yescarta targets the CD19 antigen and is primarily used for patients with large B-cell lymphoma when initial treatments fail, when cancer recurs within a year, or after at least two treatments for follicular lymphoma have not succeeded. Its significant market presence is largely due to improved survival outcomes for adults with relapsed large B-cell lymphoma. According to Gilead's 2022 data, patients treated with Yescarta had a two-year survival rate without disease progression of 40.5%, which is double that of standard treatments, and their median event-free survival was four times greater. The Carvykti segment is anticipated to experience substantial growth in the CAR T-cell therapy market. Carvykti, an autologous immunotherapy, targets B cell maturation antigen (BCMA) on cancer cells. Strong regulatory backing has facilitated its rapid development and approval; for example, on February 28, 2022, Ciltacabtagene autoleucel received approval from the US FDA for patients with relapsed or refractory multiple myeloma. Clinical trials have demonstrated impressive efficacy, with approximately 98% of participants responding to treatment and 78% showing no detectable cancer in their bone marrow or blood. The median duration of these responses lasted around 22 months, indicating a promising therapeutic option for cancer patients.
Indication Type Segment Analysis
The CAR T-cell therapy market can be analyzed based on indication types, such as Multiple Myeloma, B-cell Lymphoma, Acute Lymphoblastic Leukemia, and Others. B-cell lymphoma represents the largest segment, driven by therapies like Yescarta and Kymriah, which have received approval for treating various types of B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL). These therapies have established a robust market presence due to their effectiveness and extensive clinical use. The fastest-growing segment is Multiple Myeloma, fueled by recent advancements in CAR T-cell therapies like Carvykti and Breyanzi. These therapies specifically target multiple myeloma and are gaining traction as clinical studies demonstrate promising results in patient outcomes. The expanding indications and the ongoing development of new CAR T-cell products tailored for multiple myeloma are propelling this segment’s rapid growth, reflecting the increasing demand for effective treatments in hematological cancers. This dynamic landscape underscores the potential for CAR T-cell therapies to reshape treatment paradigms in oncology.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner market share. For instance,
- In March 2024, Bristol Myers Squibb received FDA accelerated approval for Breyanzi, a CAR T-cell therapy targeting relapsed or refractory chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) after at least two prior treatments. This marks a significant advancement in treatment options for these patients, offering a one-time infusion with the potential for durable responses and addressing an unmet medical need in a challenging disease area
- In February 2024, BioNTech and Autolus Therapeutics entered into a strategic collaboration to advance their autologous CAR T-cell toward commercialization, including a USD 200 million investment from BioNTech. The partnership will enhance BioNTech's BNT211 program and provide access to Autolus' manufacturing and targeting technologies, aiming to expand oncology treatment options and accelerate development pipelines
- In February 2024, AstraZeneca acquired Gracell Biotechnologies for approximately USD 1 billion, enhancing its cell therapy pipeline with the novel CAR-T therapy GC012F, aimed at treating multiple myeloma and autoimmune diseases
- In January 2024, AbbVie and Umoja Biopharma entered into exclusive agreements to develop in-situ generated CAR T-cell therapies for oncology using Umoja's VivoVec platform, including a focus on a CD19-directed therapy. The collaboration aims to enhance patient treatment options and streamline the CAR-T process, with Umoja potentially receiving up to USD 1.44 billion in milestones and royalties
The CAR T-cell therapy market is anticipated to experience significant growth in the upcoming years due to technological advancements, rising R&D investments, and aggressive organic and inorganic growth strategies followed by the players.
Competitive Landscape Analysis
The global CAR T-cell therapy market is marked by the presence of established and emerging players market players such as Allogene Therapeutics, Inc.; Autolus Therapeutics; Bristol-Myers Squibb Company; CARsgen Therapeutics Holdings Ltd; Cartesian Therapeutics, Inc.; Gilead Sciences, Inc.; ImmunoACT; IASO Biotherapeutics; Johnson & Johnson; JW (Cayman) Therapeutics Co Ltd; Novartis AG; and Wugen among others.
Get a sample report for competitive landscape analysis
Future Outlook of the CAR T-cell Therapy Market
The global CAR T-cell therapy market is expected to gain further momentum in the coming years due to the shift towards personalized medicine, increased awareness among healthcare professionals and development of next-generation CAR-T products. These factors collectively contribute to the growth and evolution of the CAR T-cell therapy market.
CAR T-cell Therapy Market Scope
|
Report Scope |
Details |
|
Base Year Considered |
2023 |
|
Historical Data |
2022 - 2023 |
|
Forecast Period |
2024 - 2029 |
|
Growth Rate |
CAGR 35-40% |
|
Segment Scope |
Product, Indication, Target, End User |
|
Regional Scope |
|
|
Key Companies Mapped |
Allogene Therapeutics, Inc.; Autolus Therapeutics; Bristol-Myers Squibb Company; CARsgen Therapeutics Holdings Ltd; Cartesian Therapeutics, Inc.; Gilead Sciences, Inc.; ImmunoACT; IASO Biotherapeutics; Johnson & Johnson; JW (Cayman) Therapeutics Co Ltd; Novartis AG; and Wugen; among others. |
|
Report Highlights |
Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global CAR T-cell Therapy Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global CAR T-cell Therapy Market?
-
How has COVID-19 impacted the Global CAR T-cell Therapy Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2023-2024)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- CAR T-cell Therapy Market Snapshot (2023-2029)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- The rising prevalence of cancer worldwide
- Advancements in CAR T-cell technology
- Strong pipeline of CAR T-cell therapies
- Growing strategic collaborations and partnerships
- Increase in R&D investments for new CAR T-cell therapies
- High efficacy of CAR T-cell therapy
- Restraints
- High cost of therapy
- Complex manufacturing process
- Potential for severe side effects
- Limited availability of treatment centers
- Opportunities
- Expansion into solid tumors
- Development of allogeneic CAR T-cell therapies
- Combination therapies
- Key Market Trends
- Next-generation CAR T-cells
- Combination therapies
- Manufacturing advancements
- Unmet Market Needs
- Pipeline Analysis
- Industry Speaks
- Regulatory Analysis
- Drivers
- Market Dynamics
- Global CAR T-cell Therapy Market Size & Forecast (2022-2029), By Product Type, USD Million
- Introduction
- Abecma
- Breyanzi
- Carvykti
- Yescarta
- Tecartus
- Kymriah
- Others
- Global CAR T-cell Therapy Market Size & Forecast (2022-2029), By Indication Type, USD Million
- Introduction
- Multiple Myeloma
- B-cell Lymphoma
- Acute Lymphoblastic Leukemia
- Others
- Global CAR T-cell Therapy Market Size & Forecast (2022-2029), By Target Type, USD Million
- Introduction
- BCMA
- CD19
- Others
- CAR T-cell Therapy Market Size & Forecast (2022-2029), By End User Type, USD Million
- Introduction
- Hospitals
- Long-term Care Facilities
- Specialty Centers
- Others
- Global CAR T-cell Therapy Market Size & Forecast (2022-2029), By Region, USD Million
- Introduction
- North America CAR T-cell Therapy Market Size & Forecast (2022-2029), By Country, USD Million
- US
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Canada
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Europe CAR T-cell Therapy Market Size & Forecast (2022-2029), By Country, USD Million
- UK
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Germany
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- France
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Italy
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Spain
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Rest of Europe
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Asia Pacific (APAC) CAR T-cell Therapy Market Size & Forecast (2022-2029), By Country, USD Million
- China
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Japan
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- India
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Latin America (LATAM) CAR T-cell Therapy Market Size & Forecast (2022-2029), USD Million
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- Middle East & Africa (MEA) CAR T-cell Therapy Market Size & Forecast (2022-2029), USD Million
- Market Size & Forecast, By Product Type (USD Million)
- Market Size & Forecast, By Indication Type (USD Million)
- Market Size & Forecast, By Target Type (USD Million)
- Market Size & Forecast, By End User Type (USD Million)
- China
- UK
- US
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2023)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2024)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles*
(Business Overview, Financial Performance**, Products Offered, Recent Developments)
- Allogene Therapeutics, Inc.
- Autolus Therapeutics
- Bristol-Myers Squibb Company
- CARsgen Therapeutics Holdings Ltd
- Gilead Sciences, Inc.
- ImmunoACT
- IASO Biotherapeutics
- Johnson & Johnson
- JW (Cayman) Therapeutics Co Ltd
- Novartis AG
- Other Prominent Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Long Term Care Facilities; Oncologists, Haematologist, Medical Researchers, and Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.