Cardiac Safety Services Market Size Set to Surge with Anticipated 11-12% CAGR from 2024 to 2029
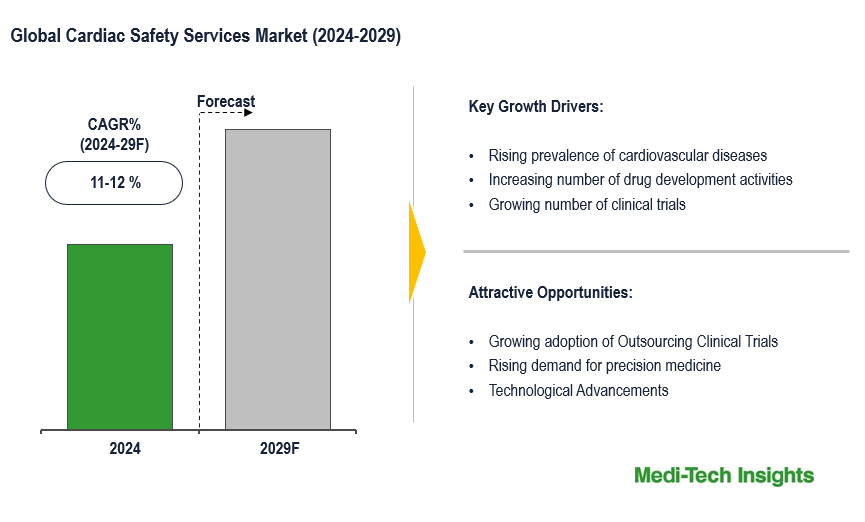
The Cardiac Safety Services Market is expected to witness a CAGR of 11-12%. The cardiac safety services market is being driven by various factors, including the pharmaceutical industry’s expansion and increasing drug development activities, the demand for precision medicine, the escalating prevalence of cardiovascular diseases mandating thorough safety assessments, advancements in real-time monitoring technologies, and the increasing utilization of biomarkers for early risk detection. Moreover, the increasing volume of clinical trials reinforces the need for extensive safety evaluations throughout the drug development continuum. To learn more about the research report, download a sample report.
Cardiac safety services refer to a range of specialized medical services and assessments to evaluate the effects of pharmaceuticals, medical devices, or other interventions on the cardiovascular system. These services are crucial during the development of new drugs, as well as in post-marketing surveillance to ensure the safety of medications already on the market. Here are some common components of cardiac safety services:
- Electrocardiography (ECG/EKG): This involves measuring the electrical activity of the heart to detect abnormalities or changes induced by drugs or medical interventions
- Holter Monitoring: This involves the continuous recording of the heart's rhythm over a period of time, typically 24 to 48 hours, using a portable device. It helps identify irregular heart rhythms that may not be captured during a standard ECG
- Event Monitoring: Similar to Holter monitoring, event monitoring involves wearing a portable device to record heart rhythms, but it is typically used for longer periods, sometimes weeks or months, to capture intermittent cardiac events
- T-wave alternans testing: This is a specialized test used to detect subtle changes in the heart's electrical activity that may indicate an increased risk of dangerous heart rhythms
- Thorough QT/QTc Studies (TQT): These are clinical trials specifically designed to evaluate the potential of a drug to prolong the QT/QTc interval.
- Risk Assessment and Mitigation Strategies: Cardiac safety services also involve evaluating the overall cardiovascular risk associated with a drug and implementing strategies to mitigate any identified risks
Safeguarding Cardiac Health: The Multi-Domain Impact of Cardiac Safety Services
Cardiac safety services are integral across diverse realms, including drug development, clinical trials, and patient care, to uphold cardiac well-being. In drug development, these services are vital for assessing potential cardiovascular impacts of new drugs or therapies through rigorous evaluations, ensuring the introduction of safe and effective treatments to the market. In clinical trials, cardiac safety services are indispensable for monitoring participants' cardiovascular health, involving electrocardiograms (ECGs) and other assessments to promptly detect abnormalities or adverse events. By closely monitoring cardiac parameters, researchers uphold participant safety and accurately evaluate investigational drugs' cardiovascular effects. Moreover, in patient care, these services are crucial for managing individuals with heart conditions or undergoing cardiac treatments. This includes various diagnostic tests like stress tests, echocardiograms, and Holter monitoring to evaluate cardiac function and track patient progress. For instance,
- In November 2023, Clario introduced its innovative AI-driven ECG Quality Score tool, aiming to bolster cardiac safety evaluations in clinical trials by offering actionable insights and potentially facilitating waivers of traditional Thorough QT studies, complementing its existing EPQT solution
- In August 2023, researchers from Philips showcased findings at the European Society of Cardiology Congress in Amsterdam, illustrating the potential utility of certain diagnostic referral codes in predicting the optimal duration of mobile telemetry or Holter monitoring for cardiology patients. This predictive approach aims to remotely monitor symptoms, potentially averting the need for additional hospitalization
Additionally, these services support optimizing medication regimens, lifestyle changes, and patient education to promote heart health and prevent cardiovascular complications. Overall, the comprehensive and proactive approach of cardiac safety services is pivotal in identifying, managing, and averting cardiac-related risks, ultimately enhancing patient safety and improving outcomes across drug development, clinical trials, and patient care.
To learn more about this report, download the PDF brochure
From Precision to Compliance: Dynamics of Cardiac Safety Services
The landscape of cardiac safety services is being shaped by several key factors. Firstly, the pharmaceutical industry's growth and the increasing number of drugs in development, drive demand for comprehensive cardiac safety assessments to ensure the safety and efficacy of new medications. With advancements in medical science, there is a growing emphasis on precision medicine, which involves tailoring treatments to individual patients based on their genetic makeup, biomarker profiles, and other factors. This personalized approach to healthcare requires comprehensive cardiac safety assessments to ensure that treatments are safe and effective for specific patient populations. Additionally, the rising burden of cardiovascular diseases worldwide highlights the need for robust cardiac safety assessments to monitor the effects of medications used in managing these conditions. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have stringent requirements for assessing the cardiac safety of new drugs. Compliance with these regulatory guidelines is driving the demand for cardiac safety services, including thorough QT/QTc studies and other assessments. For instance,
- In August 2023, Dr. Vince Clinical Research (DVCR) announced a partnership with Clario to enhance access to Clario's Early Precision QT (EPQT) methodology, enabling more accurate and cost-efficient cardiac safety data collection in early clinical trials. Through meticulous evaluation and advanced technologies, DVCR becomes an ideal candidate for complex trials, often fulfilling regulatory requirements and allowing sponsors to potentially waive Thorough QT studies
Advancements in technology, including wearable devices and digital health platforms, are revolutionizing cardiac safety assessments by enabling real-time monitoring and data collection. The expanding use of biomarkers and the emphasis on early detection and risk prediction further contribute to the growth and evolution of the cardiac safety services market. The growth of the cardiac safety services market is further propelled by the increasing trend among pharmaceutical companies to outsource clinical trial activities to specialized contract research organizations (CROs) and service providers, who provide expertise, infrastructure, and resources for conducting thorough cardiac safety assessments.
Key Constraints/Challenges
The cardiac safety services market faces numerous challenges, including stringent regulatory requirements imposed by agencies such as the FDA and EMA, which can lead to increased complexity and costs in compliance. Additionally, the complex nature of cardiac safety assessments demands specialized expertise and access to advanced technologies, making accurate data interpretation challenging, particularly for novel therapies or complex cardiovascular conditions. Financial constraints further complicate matters, as pharmaceutical companies must balance the need for comprehensive safety evaluations with budget considerations. Interpreting cardiac data collected during clinical trials or post-market surveillance requires a high level of proficiency in cardiology and statistical analysis, presenting difficulties in distinguishing drug-related effects from natural variations. Despite advancements in biomarkers and predictive modelling techniques, accurately predicting cardiac safety risks and detecting adverse events early in the drug development process remains a significant challenge, underscoring the need for ongoing research and innovation in the field.
Regional Dynamics of the Cardiac Safety Services Market
Regional segmentation analysis for cardiac safety services typically takes into account various factors influencing market dynamics across different geographical regions. In North America, the cardiac safety services market often takes the lead, primarily attributed to the presence of major pharmaceutical companies, well-developed healthcare infrastructure, and a supportive regulatory framework. Notably, the United States holds a significant market share, driven by its robust drug development pipeline and stringent regulatory standards.
In Europe, increasing research and development activities, a growing emphasis on precision medicine, and collaborations between pharmaceutical companies and research institutions contribute to the region's market growth. The Asia Pacific region is witnessing rapid expansion in the cardiac safety services market, fueled by factors such as rising healthcare expenditure, an increasing prevalence of cardiovascular diseases, and a burgeoning pharmaceutical industry. Countries like China, India, and Japan are emerging as key players, benefiting from their large patient populations, growing clinical trial activities, and improving regulatory environments. Overall, each region presents distinctive opportunities and challenges for the market, offering varying levels of market penetration and growth potential, empowering stakeholders to customize strategies to seize opportunities and tackle specific challenges within each market segment.
Competitive Landscape
Major players in the Cardiac Safety Services Market include Philips Healthcare, IQVIA, Medpace, Certara, Eurofins Scientific, SGS SA, Banook Group, Celerion, Biotrial, NEXEL Co., Ltd, Richmond Pharmacology, PhysioStim, and Clario, among others.
Get a sample report for competitive landscape analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In October 2023, Motion Equity Partners secured a majority ownership interest in Banook Group in partnership with Turenne Santé and the company's management team
- In June 2022, The Banook Group, focusing on cardiac safety, clinical imaging, and endpoint adjudication, inaugurates its first US office in Boston, Massachusetts, marking its third international expansion following its establishment in 1999, alongside headquarters in Nancy (France) and branches in Munich (Germany) and Montreal (Canada)
The Cardiac Safety Services Market is expected to gain momentum in the coming years due to the growing prevalence of cardiovascular diseases, rising number of drug development activities, technological advancements, strategic collaborations and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Cardiac Safety ServicesMarket?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Cardiac Safety Services Market?
-
How has COVID-19 impacted the Global Cardiac Safety Services Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Dynamics
- Global Cardiac Safety Services Market - Size & Forecast (2021-2028), By Services
- ECG/Holter Measurements
- Real-time Telemetry Monitoring
- Cardiovascular Imaging
- Other Services
- Global Cardiac Safety Services Market - Size & Forecast (2021-2028), By Phase
- Phase 1
- Phase 2
- Phase 3
- Global Cardiac Safety Services Market - Size & Forecast (2021-2028), By Type
- Integrated Services
- Standalone Services
- Global Cardiac Safety Services Market - Size & Forecast (2021-2028), By End User
- Bio-Pharma Companies
- CRO’s
- Other End Users
- Global Cardiac Safety Services Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Philips Healthcare
- IQVIA
- Medpace
- Certara
- Eurofins Scientific
- SGS SA
- Banook Group
- Celerion
- Biotrial
- NEXEL Co., Ltd
- Richmond Pharmacology
- PhysioStim
- Clario
- Other Players
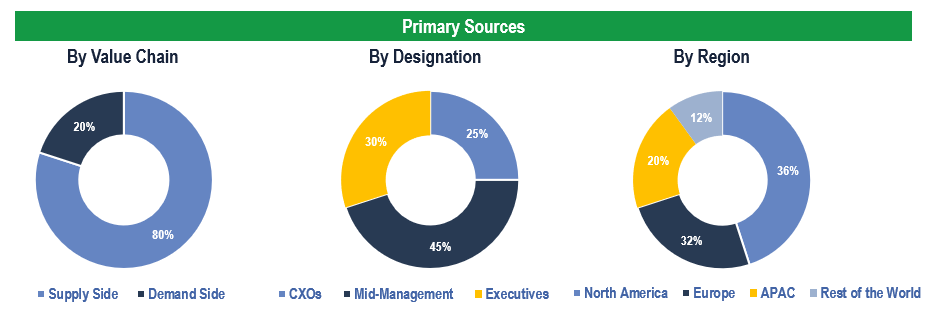
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Bio-Pharma Companies, CRO’s and Academic & Research Institutes among others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.