
Global Clinical Trial Supplies Market Size & Trends Report Segmented by Phase (Phase I, Phase II, Phase III), Product & Service (Manufacturing, Storage & Retention, Others), Therapeutic Area (Neurology, Oncology, Others), End-user & Regional Forecast to 2030
The clinical trial supplies market is projected to grow at a CAGR of ~8% over the forecast period. Key factors driving this growth include the rising number of clinical trials, increasing complexity in trial designs, and the globalization of research activities. However, market expansion faces challenges such as regulatory hurdles, logistical complexities, and high costs associated with supply chain management. To learn more about the research report, download a sample report.
Report Overview
Clinical trial supplies encompass the procurement, distribution, and management of materials necessary for conducting clinical studies. These supplies include investigational drugs, comparator medications, placebos, kits, and ancillary materials required to ensure trial integrity. The process involves packaging, labeling, blinding, and distribution logistics to maintain compliance with regulatory standards and good manufacturing practices (GMP). Effective management of clinical trial supplies is crucial to ensuring patient safety, study validity, and timely completion of trials.

To learn more about this report, download the PDF brochure
Increasing complexity of clinical trials driving market growth
The growing complexity of clinical trials is a primary factor propelling the clinical trial supplies market. Modern trials involve decentralized models, adaptive designs, and precision medicine approaches, all of which demand sophisticated supply chain solutions. The rise of multinational trials has increased the logistical burden, requiring seamless coordination across diverse regulatory frameworks and distribution networks. Additionally, the demand for biologics and personalized therapies has led to stringent temperature-controlled supply chains and real-time monitoring solutions. Sponsors and contract research organizations (CROs) are increasingly investing in just-in-time (JIT) manufacturing, demand forecasting, and advanced packaging to reduce wastage and ensure efficient trial execution. These complexities necessitate robust supply chain strategies, making the role of clinical trial supplies indispensable for the success of pharmaceutical and biotech research initiatives.
Impact of direct-to-patient (DTP) supply models on market efficiency
A significant advancement shaping the clinical trial supplies market is the adoption of direct-to-patient (DTP) supply models. This approach enables investigational drugs and trial materials to be shipped directly to participants, reducing site visits and improving patient retention. The DTP model is particularly beneficial for rare disease trials, decentralized studies, and elderly or mobility-challenged patients. It minimizes logistical bottlenecks, lowers dropout rates, and enhances trial adherence, ultimately accelerating research timelines. Additionally, DTP distribution requires robust packaging solutions, temperature-controlled logistics, and regulatory compliance to ensure product integrity. The integration of real-time tracking, remote patient monitoring, and secure delivery channels has further streamlined this model. As trials continue to globalize and decentralize, DTP supply models are expected to gain widespread adoption, optimizing clinical research efficiency and broadening patient access to innovative therapies.
To learn more about this report, download the PDF brochure
Competitive Landscape Analysis
The clinical trial supplies market is marked by the presence of established and emerging market players such as Almac Group; Biocair; Catalent Inc.; Eurofins Scientific; KLIFO; Movianto; PCI Pharma Services; Sharp Services, LLC; Thermo Fischer Scientific Inc.; UPS Healthcare; PAREXEL International Corporation among others. Some key strategies market players adopt include strategic partnerships & collaborations, and geographic expansion.
Report Scope
| Report Metric | Details |
| Base Year Considered | 2024 |
| Historical Data | 2023 - 2024 |
| Forecast Period | 2025 – 2030 |
| Growth Rate | 8% |
| Market Drivers |
|
| Attractive Opportunities |
|
| Segment Scope | Phase, Product & Service, Therapeutic Area, and End-user |
| Regional Scope |
|
| Key Companies Mapped | Almac Group; Biocair; Catalent Inc.; Eurofins Scientific; KLIFO; Movianto; PCI Pharma Services; Sharp Services, LLC; Thermo Fischer Scientific Inc.; UPS Healthcare; PAREXEL International Corporation among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
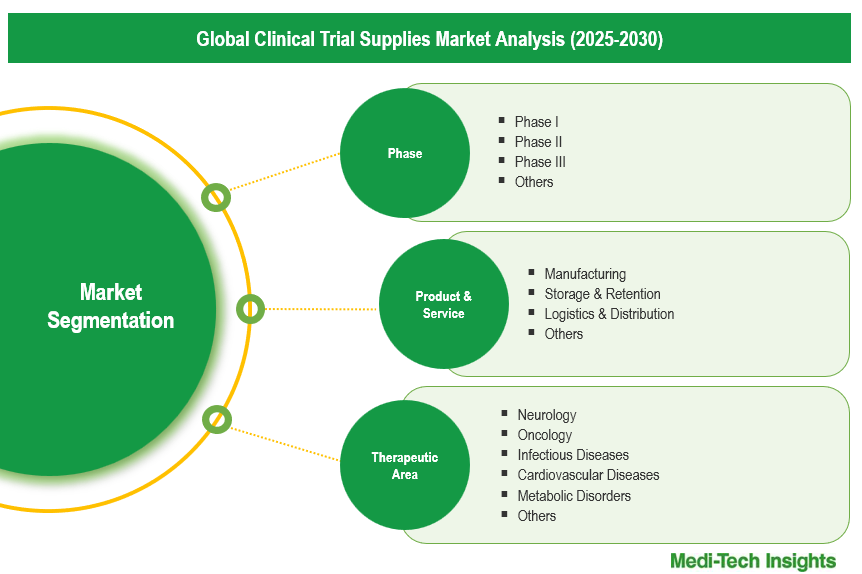
Global Clinical Trial Supplies Market Segmentation
This report by Medi-Tech Insights provides the size of the global clinical trial supplies market at the regional- and country-level from 2023 to 2030. The report further segments the market based on phase, product & service, therapeutic area and end-user.
Market Size & Forecast (2023-2030), By Phase, USD Million
- Phase I
- Phase II
- Phase III
- Others
Market Size & Forecast (2023-2030), By Product & Service, USD Million
- Manufacturing
- Storage & Retention
- Logistics & Distribution
- Others
Market Size & Forecast (2023-2030), By Therapeutic Area, USD Million
- Neurology
- Oncology
- Infectious Diseases
- Cardiovascular Diseases
- Metabolic Disorders
- Others
Market Size & Forecast (2023-2030), By End-user, USD Million
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutions
- Others
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
Key Strategic Questions Addressed
- What is the market size & forecast of the clinical trial supplies market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the clinical trial supplies market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2025-2026)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- Clinical Trial Supplies Market Snapshot (2025-2030)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Rising clinical trials with increasing drug development
- Growing adoption of biologics and personalized medicine
- Expansion of decentralized clinical trials
- Rising investments in cold chain logistics for temperature-sensitive biologics
- Increasing outsourcing to CROs and specialized supply providers
- Restraints
- High logistics and storage costs for biologics and temperature-sensitive drugs
- Regulatory and compliance challenges due to varying country-specific requirements
- Supply chain disruptions from geopolitical instability and raw material shortages
- Opportunities
- Advancements in just-in-time (JIT) manufacturing optimizing supply efficiency
- Integration of blockchain in supply chain management
- Higher demand for comparator and reference drugs
- Growth of trials in cost-effective emerging markets
- Key Market Trends
- Growth in direct-to-patient (DTP) distribution models improving accessibility
- Innovations in temperature-controlled packaging
- Unmet Market Needs
- Industry Speaks
- Drivers
- Market Dynamics
- Global Clinical Trial Supplies Market Size & Forecast (2023-2030), By Phase, USD Million
- Introduction
- Phase I
- Phase II
- Phase III
- Others
- Global Clinical Trial Supplies Market Size & Forecast (2023-2030), By Product & Service, USD Million
- Introduction
- Manufacturing
- Storage & Retention
- Logistics & Distribution
- Others
- Global Clinical Trial Supplies Market Size & Forecast (2023-2030), By Therapeutic Area, USD Million
- Introduction
- Neurology
- Oncology
- Infectious Diseases
- Cardiovascular Diseases
- Metabolic Disorders
- Others
- Global Clinical Trial Supplies Market Size & Forecast (2023-2030), By End-user, USD Million
- Introduction
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutions
- Others
- Global Clinical Trial Supplies Market Size & Forecast (2023-2030), By Region, USD Million
- Introduction
- North America Clinical Trial Supplies Market Size & Forecast (2023-2030), By Country, USD Million
- US
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Canada
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- US
- Europe Clinical Trial Supplies Market Size & Forecast (2023-2030), By Country, USD Million
- UK
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Germany
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- France
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Italy
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Spain
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Europe
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- UK
- Asia Pacific (APAC) Clinical Trial Supplies Market Size & Forecast (2023-2030), By Country, USD Million
- China
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Japan
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- India
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- China
- Latin America (LATAM) Clinical Trial Supplies Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Middle East & Africa (MEA) Clinical Trial Supplies Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Phase (USD Million)
- Market Size & Forecast, By Product & Service (USD Million)
- Market Size & Forecast, By Therapeutic Area (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2024)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2025)
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles*
(Business Overview, Financial Performance**, Offerings, Recent Developments)
- Almac Group
- Biocair
- Catalent Inc.
- Eurofins Scientific
- KLIFO
- Movianto
- PCI Pharma Services
- Sharp Services, LLC
- Thermo Fischer Scientific Inc.
- UPS Healthcare
- PAREXEL International Corporation
- Other Prominent Players
Note: *Indicative list
**For listed companies
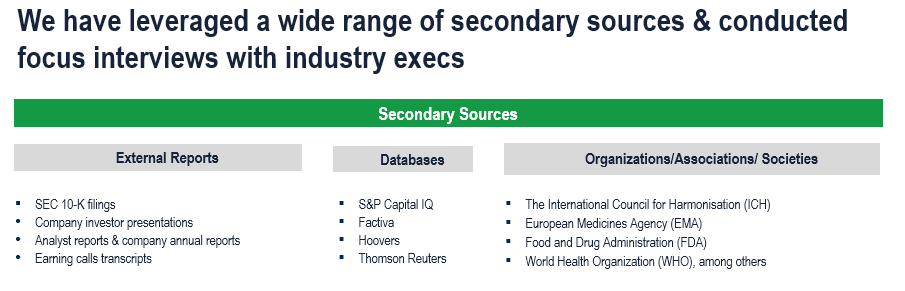
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
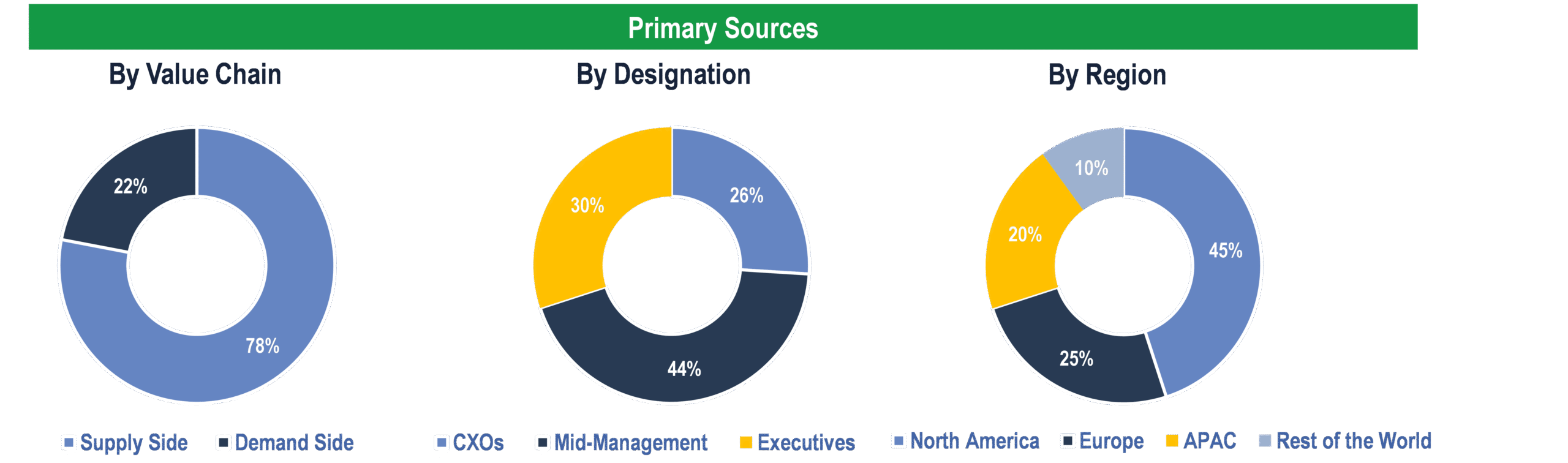
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders from Pharmaceutical & Biotechnology Companies, CROs and Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data



