Global eClinical Solutions Market Size & Trends Report by Products (EDC, CTMS, RTSM, eCOA, Safety), Deployment Mode (Web & Cloud Based, On-premise), Clinical Trial Phase, End Users (Pharma, Biotech) Analysis & Regional Forecasts to 2029
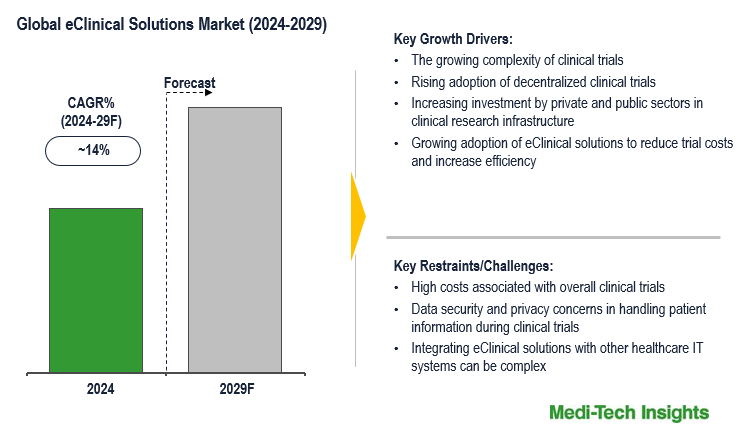
The global eClinical solutions market is expected to grow at a CAGR of ~14% in the next five years. The growing complexity of clinical trials, rising adoption of decentralized clinical trials, a surge in clinical trial outsourcing, increasing investment by private and public sectors in clinical research infrastructure, and growing adoption of eClinical solutions to reduce trial costs and improve efficiency are some key factors driving the eClinical solutions market. To learn more about the research report, download a sample report.
Report Overview
eClinical solutions include a broad array of digital tools and software designed to simplify and enhance various aspects of clinical trial processes in the pharmaceutical, biotech, and medical device industries. By leveraging technology, these solutions streamline and improve data collection, management, and analysis in clinical trials, ensuring regulatory compliance, boosting efficiency, and aiding in informed decision-making.
To learn more about this report, download the PDF brochure
Adoption of Decentralized Clinical Trials (DCTs) in clinical research propels market growth
The increasing adoption of decentralized clinical trials (DCTs) marks a significant transformation in clinical research, driven by advances in digital health technology, patient-centered models, and the need for more flexible trial designs. eClinical tools like electronic patient-reported outcomes (ePRO), telemedicine, and mobile data collection enable remote trial management, making it easier to engage participants and enhancing the overall patient experience. By removing geographic and logistical barriers, DCTs allow for broader inclusion of patients from rural or underserved areas, resulting in more diverse and representative study populations. The convenience of participating from home leads to higher adherence and retention rates, which helps preserve data integrity and reduces costs related to dropouts. Through greater accessibility, increased efficiency, and a focus on patient-centered practices, DCTs have the potential to redefine the future of clinical trials, making research more inclusive and attuned to patient needs.
The advantages of utilizing eClinical solutions in clinical trials fuel its demand
Clinical trials are becoming more complex due to the nature of new therapies, targeted treatments, and personalized medicine. eClinical solutions help streamline data collection, analysis, and management in multi-phase, multinational trials that require extensive coordination and compliance with varying regulatory standards. The benefits of using eClinical solutions are extensive and multifaceted, including:
- Improved Data Accuracy: Electronic data capture (EDC) and electronic case report form (eCRF) systems significantly reduce errors and inconsistencies in data entry. Real-time validation checks are integral to maintaining high data quality.
- Enhanced Efficiency: By streamlining processes and automating workflows, eClinical solutions help accelerate trial timelines, from patient recruitment through data analysis.
- Data Security: Built-in data security features protect patient and research data, ensuring compliance with regulatory standards.
- Regulatory Compliance: eClinical solutions are essential for meeting stringent regulatory requirements and support the creation of audit trails to ensure accountability.
To learn more about this report, download the PDF brochure
Competitive Landscape Analysis
The global eClinical solutions market is marked by the presence of innovative and emerging market players such as Oracle, Parexel International Corporation, Medidata, Signant Health, Clario (ERT and Bioclinica), eClinicalWorks, eClinical Solutions, IQVIA, Castor EDC, ArisGlobal, Veeva Systems, among others. Some of the key strategies adopted by the market players include mergers and acquisitions, strategic partnerships, and collaborations.
Report Scope
| Report Metric | Details |
| Base Year Considered | 2023 |
| Historical Data | 2022 – 2023 |
| Forecast Period | 2024 – 2029 |
| Growth Rate | CAGR of ~14% |
| Market Drivers |
|
| Key Market Trends |
|
| Segment Scope | Product, Deployment Mode, Clinical Trial Phase, End User |
| Regional Scope |
|
| Key Companies Mapped | Oracle, Parexel International Corporation, Medidata, Signant Health, Clario (ERT and Bioclinica), eClinicalWorks, eClinical Solutions, IQVIA, Castor EDC, ArisGlobal, Veeva Systems, among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Market Segmentation
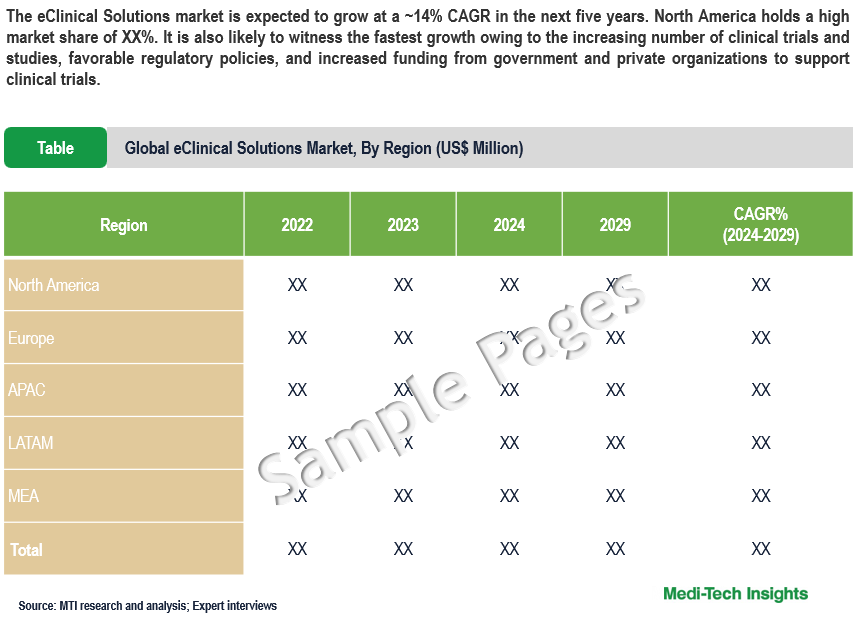
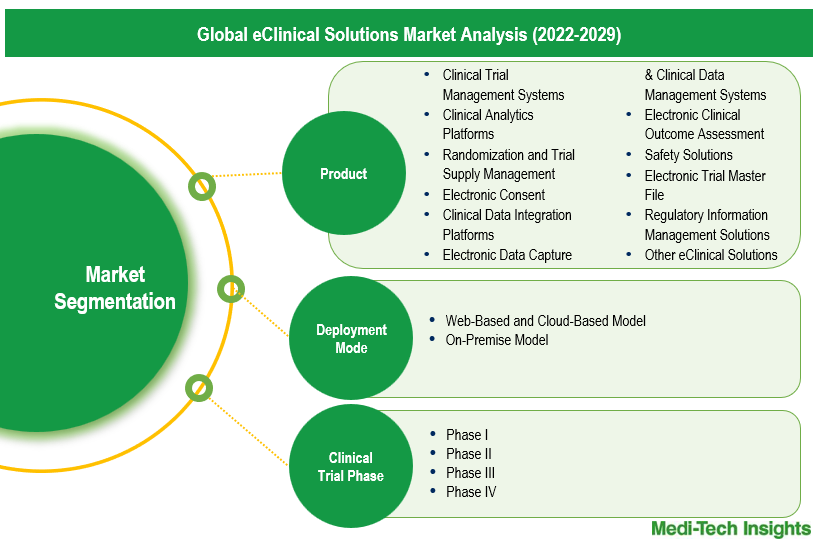
This report by Medi-Tech Insights provides the size of the global eClinical solutions market at the regional- and country level from 2022 to 2029. The report further segments the market based on product, deployment mode, clinical trial phase, and end user.
- Market Size & Forecast (2022-2029), By Product, USD Million
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platforms
- Randomization and Trial Supply Management (RTSM)
- Electronic Consent (eConsent)
- Clinical Data Integration Platforms
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trial Master File (eTMF)
- Regulatory Information Management Solutions (RIMS)
- Other eClinical Solutions
- Market Size & Forecast (2022-2029), By Deployment Mode, USD Million
- Web-Based and Cloud-Based Model
- On-Premise Model
- Market Size & Forecast (2022-2029), By Clinical Trial Phase, USD Million
- Phase I
- Phase II
- Phase III
- Phase IV
- Market Size & Forecast (2022-2029), By End User, USD Million
- Hospitals/Healthcare providers
- Contract Research Organizations (CROs)
- Academic Research Institutes
- Pharma & Biotech Companies
- Medical Device Manufacturers
- Consulting Service Providers
- Government Organizations
- Other End Users
- Market Size & Forecast (2022-2029), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
- North America
Key Strategic Questions Addressed
- What is the market size & forecast of the eClinical solutions market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the eClinical solutions market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market?
- Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2024-2025)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- eClinical Solutions Market Snapshot (2024-2029)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- The growing complexity of clinical trials
- Rising adoption of decentralized clinical trials
- Increasing investment by private and public sectors in clinical research infrastructure
- Growing adoption of eClinical solutions to reduce trial costs and increase efficiency
- Restraints
- High costs associated with overall clinical trials
- Data security and privacy concerns in handling patient information during clinical trials
- Integrating eClinical solutions with other healthcare IT systems can be complex
- Opportunities
- Key Market Trends
- Leveraging integration of artificial intelligence and machine learning
- Growing demand for cloud-based solutions
- Development of wearable and mobile health technologies
- Growing usage of real-world data (RWD) and real-world evidence (RWE)
- Unmet Market Needs
- Industry Speaks
- Drivers
- Market Dynamics
- Global eClinical Solutions Market Size & Forecast (2022-2029), By Product, USD Million
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platforms
- Randomization and Trial Supply Management (RTSM)
- Electronic Consent (eConsent)
- Clinical Data Integration Platforms
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trial Master File (eTMF)
- Regulatory Information Management Solutions (RIMS)
- Other eClinical Solutions
- Global eClinical Solutions Market Size & Forecast (2022-2029), By Deployment Mode, USD Million
- Web-Based and Cloud-Based Model
- On-Premise Model
- Global eClinical Solutions Market Size & Forecast (2022-2029), By Clinical Trial Phase, USD Million
- Phase I
- Phase II
- Phase III
- Phase IV
- Global eClinical Solutions Market Size & Forecast (2022-2029), By End User, USD Million
- Hospitals/Healthcare providers
- Contract Research Organizations (CROs)
- Academic Research Institutes
- Pharma & Biotech Companies
- Medical Device Manufacturers
- Consulting Service Providers Companies
- Government Organizations
- Other End Users
- Global eClinical Solutions Market Size & Forecast (2022-2029), By Region, USD Million
- Introduction
- North America eClinical Solutions Market Size & Forecast (2022-2029), By Country, USD Million
- US
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Canada
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- US
- Europe eClinical Solutions Market Size & Forecast (2022-2029), By Country, USD Million
- UK
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Germany
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- France
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Italy
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Spain
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Rest of Europe
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- UK
- Asia Pacific (APAC) eClinical Solutions Market Size & Forecast (2022-2029), By Country, USD Million
- China
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Japan
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- India
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- China
- Latin America (LATAM) eClinical Solutions Market Size & Forecast (2022-2029), USD Million
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Middle East & Africa (MEA) eClinical Solutions Market Size & Forecast (2022-2029), USD Million
- Market Size & Forecast, By Product (USD Million)
- Market Size & Forecast, By Deployment Mode (USD Million)
- Market Size & Forecast, By Clinical Trial Phase (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2023)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2024)
- Partnerships, Agreements, & Collaborations
- New Product Launches
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles*
(Business Overview, Financial Performance**, Products & Services Offered, Recent Developments)
- Signant Health
- Oracle
- Parexel International Corporation
- Medidata Soultions
- Clario (ERT and Bioclinica)
- Castor EDC
- ArisGlobal
- eClinical Solutions
- IQVIA
- Veeva Systems
- Other Prominent Players
Note: *Indicative list
**For listed companies
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
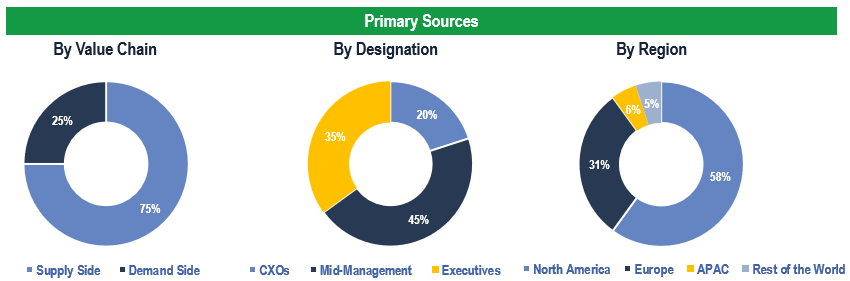
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers, Others
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants, Others
Demand Side Stakeholders:
- Hospitals/Healthcare providers, Contract Research Organizations (CROs), Academic Research Institutes, Pharma & Biotech Organizations, Medical Device Manufacturers, Consulting Service Companies, Government Organizations, Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, the Internal Knowledge Repository, and the Company’s Sales Data.