Fetal Monitoring Devices Market Size, Share, Trends, Demand & Growth 2027
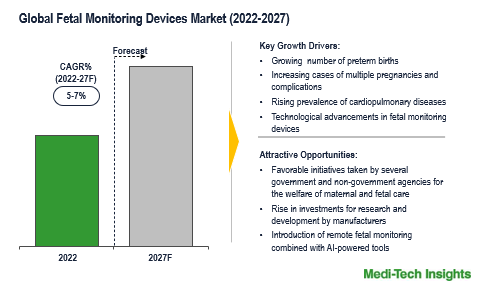
The Global Fetal Monitoring Devices Market is expected to grow at a rate of 5-7 % by 2027. Growing cases of preterm births, congenital birth defects, hemorrhage, and rapid advancements in technology are some of the key factors driving the market growth.
Fetal monitoring devices are diagnostic tools used to monitor the vital parameters of fetal throughout pregnancy, labor, and delivery. During the first trimester, it is primarily used for monitoring fetal heart rate and fetal movement. These systems help in identifying any disorders or birth defects, monitor fetal health, and track uterine contractions to ensure the safe birth of the baby.
Despite a Slowdown in the Demand Owing to Covid-19, the Fetal Monitoring Devices Market Stays Resilient
The Covid-19 Pandemic changed the complete socio-economic scenario in the healthcare sector. Due to safety reasons, the hospitals had to limit the volume of patients, reducing the fetal monitoring procedures. During the pandemic, healthcare providers delayed or rescheduled fetal monitoring procedures to minimize the risk of patients getting exposed to Covid-19. Hence, during the pandemic, there was a substantial decrease in the number of fetal monitoring procedures. However, to overcome these barriers, companies launched several innovative tools to help monitor pregnant women during COVID-19.
For instance, in June 2020, Philips launched an obstetrics monitoring solution to support clinicians and expectant mothers during the COVID-19 pandemic. The firm launched Avalon CL Fetal and Maternal Pod and Patch which reduces unnecessary physical interactions between clinicians and patients, which is of particular importance during the COVID-19 pandemic.
“Covid-19 has intensified the need for mobile solutions during pregnancy. Remote monitoring especially during labor offers several benefits such as comfort, mobility, and flexibility to expectant mothers.” -General Manager, Monitoring & Analytics, Health Technology Company, U.S.
Technological Advancements in Fetal Monitoring Devices Drives the Global Fetal Monitoring Devices Market
The growing demand for fetal monitoring devices/systems can be attributed to the rising prevalence of cardiopulmonary diseases, post-term pregnancy, multiple pregnancies, preterm births, initiatives taken by the government for the welfare of maternal and fetal health, and new innovations in fetal monitoring devices market.
For instance,
- In November 2022, Philips announced the global launch of the Ultrasound Compact 5000 series. The system is portable, versatile and offers multiple care settings for different types of examinations with superb image quality
- In July 2022, G.E. Healthcare market leader in Women’s Health portfolio announced the launch of its latest Ultrasound Innovation Voluson Expert 22 which includes a graphic-based beam former technology, AI-powered tool, and customized touch panel.
Rising Cases of Preterm Births Spurs the Growth of Fetal Monitoring Devices Market
One of the key factors which are driving the fetal monitoring devices market is an increase in the number of preterm births. Research findings suggest that key reasons for preterm births are hypertension, hemorrhage, and multiple pregnancies. The rising number of preterm births has also accelerated the adoption of advanced technology in the fetal monitoring devices market. Technology advancements and rising awareness among the people are propelling the growth of fetal monitoring devices market.
For instance,
- In July 2022, Taiwanese tech startup Cubo Ai launched a new product to augment its award-winning Cubo Ai Plus Smart Baby Monitor - the AI Sleep Sensor Pad. The new addition is designed to detect the baby's breathing motion.
Key Market Challenges: Fetal Monitoring Devices Market
The high costs of fetal monitoring devices, along with-it the availability of refurbished monitors in the market, the stringent government policies, and the lack of skilled professionals will be expected to adversely impact the growth of the fetal monitoring devices market in the upcoming years.
North America Accounts for the Largest Share of the Global Fetal Monitoring Devices Market
North America holds the largest market share of the fetal monitoring devices market. This can be mainly attributed due to rising number of preterm births, increasing maternal age, obesity & smoking, favourable government initiatives related to maternal and fetal health, growing availability of experts and neonatal intensive care units (NICUs), presence of key players and the developed healthcare system in the region. However, the Asia-Pacific region is expected to grow with the highest CAGR in the forecast period. Factors such as low-weight births, constant development in the healthcare sector, a rising number of marital-age individuals, an increase in the number of infertility treatments, rise in demand for advanced fetal monitoring devices are some of the key factors driving the Asia-Pacific market.
Competitive Landscape Analysis: Fetal Monitoring Devices Market
The key players operating in the global fetal monitoring devices market are listed as below:-
- Medtronic PLC
- Koninklijke Philips NV
- GE Company (GE Healthcare)
- Siemens Healthineers, among others.
Organic and Inorganic Growth Strategies Adopted by the Leading Players to Establish Their Foothold in the Fetal Monitoring Devices Market
All major players operating in the global fetal monitoring devices market are adopting both organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner a larger market share.
For instance,
- In February 2023, GE Healthcare acquired Caption Health. Captions Health Inc., AI applications combined with G.E. Healthcare’s ultrasound devices will enhance ultrasound imaging, diagnosis, patient care and ultimately reduce the cost.
- In March 2022, Philips announced that Collaboration Live has received U.S. FDA 510(k) clearance for remote diagnostic use to an additional mobile platform. Collaboration Live is an integrated tele-ultrasound technology that will allow real-time communication between clinicians and patients during remote ultrasound imaging on Philips U.S. EPIQ or Affinity to a compatible remote PC or mobile device.
The global fetal monitoring devices market is a growing market that is expected to gain further momentum in the coming years due to a strong emphasis on developing new wireless and non-invasive monitors, investment in R&D to introduce several advanced products, and aggressive organic and inorganic growth strategies followed by the leading market players.
Key Strategic Questions Addressed in this Report
- What is the market size & forecast for the fetal monitoring devices market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the fetal monitoring devices market?
- How has Covid-19 impacted the fetal monitoring devices market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the fetal monitoring devices market?
- What is the investment landscape of fetal monitoring devices market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the fetal monitoring devices market?
- What are the key strategies adopted by players in fetal monitoring devices market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2. Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunities
3.1.4. Market Trend
3.2. Industry Speaks
3.3. Technological Advancements
4. COVID-19 Impact on Fetal Monitoring Devices Market
5. Snapshot: Reimbursement Landscape
6. Global Fetal Monitoring Devices Market - Size & Forecast (2019-2027), By Product
6.1. Fetal Monitors
6.2. Ultrasound Devices
6.3. Uterine Contraction Monitors
6.4. Fetal Doppler Devices
6.5. Fetal Electrodes
6.6. Telemetry Devices
6.7. Accessories & Consumables
6.8. Other Products
7. Global Fetal Monitoring Devices Market - Size & Forecast (2019-2027), By Portability
7.1. Portable Systems
7.2. Non-portable Systems
8. Global Fetal Monitoring Devices Market - Size & Forecast (2019-2027), By Application
8.1. Antepartum
8.2. Intrapartum
9. Global Fetal Monitoring Devices Market - Size & Forecast (2019-2027), By End-User
9.1. Hospitals
9.2. Obstetrics and Gynaecology Clinics
9.3. Other End-users
10. Global Fetal Monitoring Devices Market - Size & Forecast (2019-2027), By Region
10.1. North America (U.S. & Canada)
10.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
10.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
10.4. Rest of the World (Latin America, Middle East & Africa)
11. Competitive Landscape
11.1. Key Players and their Competitive Positioning
11.1.1. Market Share Analysis (2022)
11.1.2. Segment-wise Player Mapping
11.2. Key Strategies Assessment, By Player (2020-2022)
11.2.1. New Product & Service Launches
11.2.2. Partnerships, Agreements, & Collaborations
11.2.3. Mergers & Acquisitions
11.2.4. Geographic Expansion
12. Key Companies Scanned (Indicative List)
12.1. Cardinal Health
12.2. Philips
12.3. GE Healthcare
12.4. Siemens Healthineers
12.5. Fujifilm
12.6. Huntleigh Healthcare (Part of Arjo)
12.7. CooperSurgical
12.8. CONTEC Medical
12.9. Neoventa Medical
12.10. Bionet
12.11. Other Prominent Players
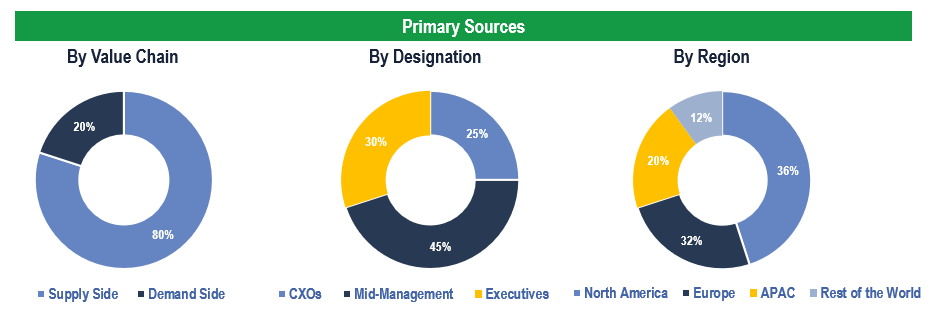
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Obstetrics and Gynecology Clinics, and Other End Users.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.