HIV Diagnostics Market Size, Share and Innovative Trends and Transformative Forces to 2028
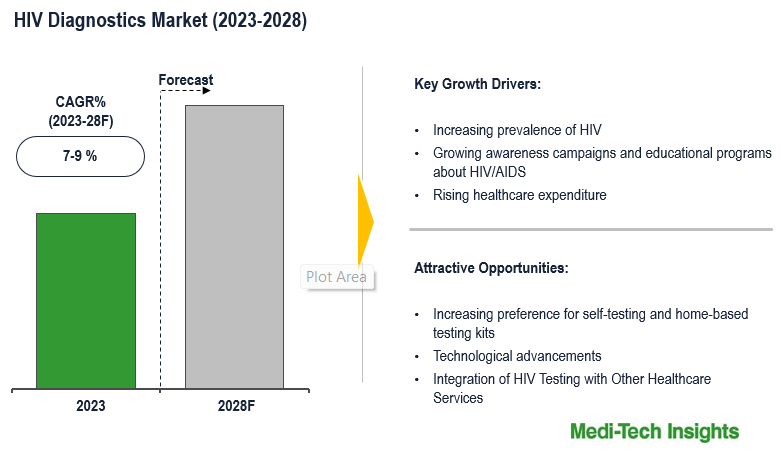
The HIV Diagnostics Market is anticipated to experience a growth rate of 7-9% between 2023 and 2028. The market’s growth is propelled by several factors, including the escalating prevalence of HIV infection, continuous technological advancements in diagnostic technologies, a conducive regulatory environment, and the development of multi-analyte platforms. These factors collectively contribute to the positive trajectory of the HIV Diagnostics Market, reflecting the ongoing efforts to enhance diagnostic precision, accessibility, and efficiency in addressing the challenges posed by HIV/AIDS. To learn more about the research report, download a sample report.
HIV diagnostics encompass the methods and procedures employed to identify the presence of the human immunodeficiency virus (HIV) in an individual's body. Timely and accurate HIV diagnostics are paramount for early detection, treatment initiation, and the prevention of further transmission. Various diagnostic tests are available for detecting HIV infection, each serving specific purposes in different settings.
The types of HIV diagnostics include antibody tests, antigen/antibody tests, and nucleic acid tests (NATs). Antibody tests, such as Enzyme Immunoassay (EIA) or Enzyme-Linked Immunosorbent Assay (ELISA), detect antibodies produced by the immune system in response to HIV infection and are commonly used as screening tests. Antigen/antibody tests detect both HIV antibodies and antigens, such as the p24 protein, allowing for earlier identification of infections compared to antibody tests alone. Nucleic acid tests (NATs), such as Polymerase Chain Reaction (PCR), identify the genetic material (RNA or DNA) of the virus and are highly sensitive, making them useful for early detection, particularly during the window period.
These diagnostic methods play a crucial role in HIV prevention, treatment, and management by enabling healthcare professionals to promptly diagnose HIV infection, initiate appropriate interventions, and prevent further transmission of the virus.
From Prevalence to Precision: Factors Shaping the HIV Diagnostics Landscape
The expansion of the HIV diagnostics market is driven by several key factors that contribute to its growth trajectory. One significant factor is the ongoing prevalence of HIV/AIDS globally, necessitating a continual demand for diagnostic tests to address this persistent health challenge. Advances in technology have led to the development of innovative diagnostic tools with improved sensitivity, specificity, and ease of use. These include rapid diagnostic tests (RDTs), molecular diagnostics, and novel biomarker detection methods, enhancing the accuracy and efficiency of HIV diagnosis. Rising awareness about HIV/AIDS and the importance of early detection, coupled with screening programs initiated by governments, NGOs, and healthcare organizations, have led to increased testing uptake among individuals, fostering market growth. Moreover, improvements in healthcare infrastructure, particularly in developing regions, have facilitated better access to diagnostic services, contributing to the overall expansion of the market. These factors collectively underscore the dynamic nature of the HIV diagnostics market, which is poised for further evolution with ongoing advancements in technology and healthcare delivery systems. For instance,
- In December 2022, Gilead Sciences, Inc. revealed that Sunlenca (lenacapavir), alongside other antiretroviral (ARV), received approval from the U.S. Food and Drug Administration (FDA) for treating HIV-1 infection in heavy treatment-experienced (HTE) adults with multi-drug resistant (MDR) HIV-1 infection
- In February 2022, ViiV Healthcare announced the approval by the US Food and Drug Administration (FDA) of Cabenuva (cabotegravir, rilpivirine) for the treatment of HIV-1 in virologically suppressed adults, with dosing intervals extended to every two months
To learn more about this report, download the PDF brochure
Expanding Horizons: Developments and Initiatives in HIV Diagnostics
The HIV diagnostics market is witnessing significant developments, particularly in the domain of point-of-care testing (POCT). The rise of POCT for HIV diagnostics is driven by its rapid turnaround time, facilitating early diagnosis and intervention, a crucial advantage, especially in resource-constrained settings with limited access to traditional laboratory facilities. There is a concerted effort to expand HIV diagnostics markets in developing regions with a high burden of HIV/AIDS. Governments, NGOs, and international entities are investing in initiatives aimed at improving access to diagnostic services, thereby stimulating market growth in these regions. For instance,
- In January 2022, Vela Diagnostics revealed that its Sentosa SQ HIV-1 Genotyping Assay has gained reimbursement coverage from The Centers for Medicare and Medicaid (CMS) in the US. This assay significantly decreases technician involvement compared to conventional methods using Sanger sequencing, necessitating less than 2 hours of hands-on time from sample collection to report generation
Integration of HIV diagnostics into existing healthcare frameworks, such as antiretroviral therapy (ART) programs and maternal and child health services, streamlines early detection and access to care, fostering improved patient outcomes and expanding market reach. Diversification of HIV testing services beyond conventional healthcare settings to community-based, outreach, and non-clinical settings like pharmacies and mobile clinics opens up new avenues for market expansion, enhancing accessibility and convenience for individuals seeking testing services. Additionally, the adoption of multiplex testing platforms capable of detecting multiple infections concurrently, including HIV, is gaining momentum, particularly in settings where co-infections are prevalent, further propelling growth within the HIV diagnostics market.
HIV Diagnostics Market: Key Constraints/Challenges
The HIV diagnostic market encounters various challenges that can hinder its efficiency and expansion. One significant obstacle is the high cost of diagnostic tests, particularly with newer and more advanced technologies, potentially limiting widespread testing initiatives and early detection efforts. Additionally, pediatric testing poses unique challenges, including the need for specialized methods and the risk of false-positive or false-negative results, requiring improved access and accurate diagnosis for timely interventions, especially in preventing mother-to-child transmission. Moreover, the emergence of drug-resistant HIV strains presents diagnostic difficulties as traditional methods may fail to detect these strains accurately, emphasizing the importance of ongoing surveillance to inform treatment decisions and effectively manage HIV infections. Addressing these challenges requires collaborative efforts to enhance access, accuracy, and affordability of diagnostic testing while adapting to evolving HIV strains and pediatric testing needs.
Regional Segmentation of the Autoimmune Disease Diagnosis Market
In North America, well-established healthcare infrastructure, technological advancements, and significant investments in HIV/AIDS prevention and treatment programs contribute to a robust market. This region also benefits from, stringent regulatory frameworks, and government initiatives aimed at controlling HIV/AIDS spread. Similarly, Europe showcases advanced healthcare systems, facilitating widespread access to diagnostic services. The market here thrives on increasing awareness, favorable reimbursement policies, and the presence of prominent market players focusing on innovative diagnostic technologies.
In contrast, the Asia Pacific region faces a significant burden of HIV/AIDS, especially in countries like India, China, and Thailand. Changing socioeconomic conditions and demographics are contributing to a rise in HIV cases in these nations. Moreover, heightened investments in healthcare facilities and awareness initiatives are driving market expansion in the Asia Pacific region.
HIV Diagnostics Market: Competitive Landscape
Some of the key players operating in the market include Siemens Healthineers, F. Hoffmann-La Roche Ltd, Abbott Laboratories, Danaher Corporation, Thermo-Fisher Scientific Inc., Merck KGaA, Becton, Dickinson & Company, Hologic Inc., Gilead Sciences Inc., Bio-Rad Laboratories, Qiagen and Mylan Inc among others.
Get a Sample Report for Competitive Landscape Analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In August 2023, The Global Fund revealed a groundbreaking agreement with generic pharmaceutical companies aimed at substantially reducing the cost of a state-of-the-art HIV medication, a development expected to have a life-saving impact. The Global Fund, established in 2002 to combat AIDS, tuberculosis, and malaria, stated that this deal would enable the provision of the advanced TLD pill at less than $45 per person annually, marking a significant milestone in access to HIV treatment
- In August 2021, Weill Cornell Medicine received a $28.5 million Martin Delaney Collaboratory grant from the National Institutes of Health to spearhead a multi-institutional initiative focused on discovering a cure for HIV. Led by Dr Brad Jones, the program, named REACH: Research Enterprise to Advance a Cure for HIV, will be based at Weill Cornell Medicine and aims to advance research efforts in this field
The HIV diagnostics market is expected to gain momentum in the coming years due to the rising incidences of HIV infections, growing awareness about HIV/AIDS and the importance of early detection, technological advancements, and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global HIV Diagnostics Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global HIV Diagnostics Market?
-
How has COVID-19 impacted the Global HIV Diagnostics Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Dynamics
- Global HIV Diagnostics Market - Size & Forecast (2021-2028), By Product Type
- Consumables
- Instruments
- Software & Services
- Other Products
- Global HIV Diagnostics Market - Size & Forecast (2021-2028), By Test Type
- Antibody Test
- Viral Load Tests
- CD4 Count Tests
- Early Infant Diagnosis Tests
- Other Tests
- Global HIV Diagnostics Market - Size & Forecast (2021-2028), By End User
- Hospitals
- Clinical Laboratories
- Blood Banks
- Academic & Research Institutes
- Other End Users
- Global HIV Diagnostics Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Siemens Healthineers
- Hoffmann-La Roche Ltd
- Abbott Laboratories
- Danaher Corporation
- Thermo-Fisher Scientific Inc.
- Merck KGaA
- Becton, Dickinson & Company
- Hologic Inc.
- Gilead Sciences Inc.
- Bio-Rad Laboratories
- Qiagen
- Mylan Inc
- Other Players
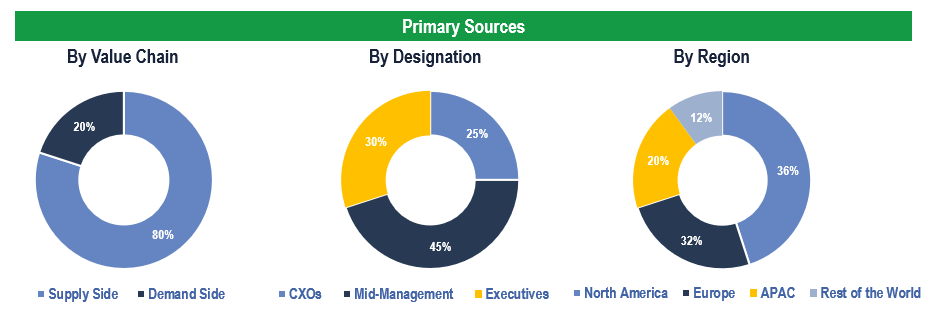
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Clinical Laboratories, Blood Banks and Academic & Research Institutes among others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.