Neonatal & Fetal (Labor & Delivery) Care Equipment Market Size, Demand, Industry Growth and Forecast to 2028
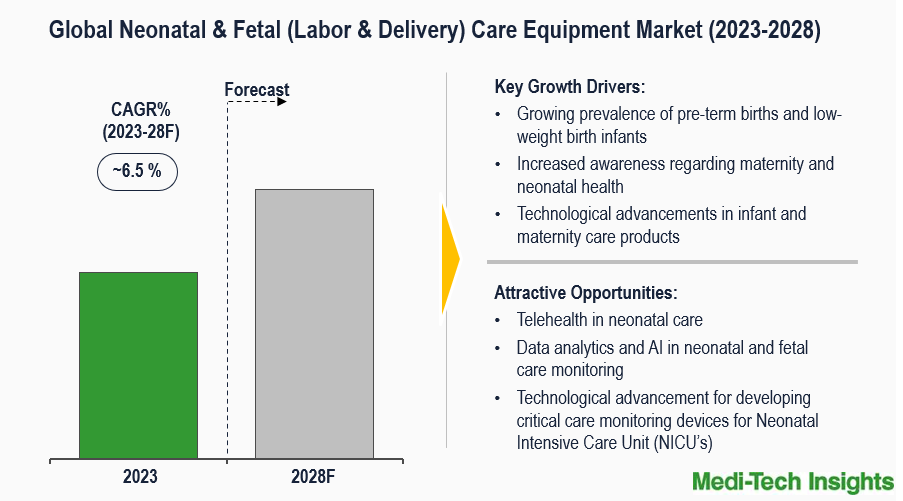
The Neonatal & Fetal (Labor & Delivery) Care Equipment Market is expected to witness a growth rate of ~6.5% by 2028. The key factors driving the market are the prevalence of pre-term births, increased awareness regarding maternity and neonatal health, a rise in the number of low-weight birth infants, and technological advancements in infant and maternity care products. To learn more about the research report, fill out a quick inquiry for a sample report.
Neonatal and fetal (Labor and delivery) Care Equipment refers to a range of medical devices designed for the specialized care of newborns and infants, as well as monitoring and support during labor and delivery. These essential tools encompass a variety of equipment such as incubators, radiant warmers, fetal monitors, and neonatal ventilators, aimed at ensuring the well-being of both mothers and their newborns.
Adoption of Neonatal & Fetal Monitoring Equipment to Reduce Stillbirths, Pre-term Births & Birth Defects Fuels the Demand
In 2021, approximately 1.9 million stillbirths, defined as babies born with no signs of life at 28 weeks of pregnancy or later, occurred globally. The latest data reveals that 1 in 72 total births worldwide resulted in a stillborn baby, equating to one every 17 seconds. Additionally, around 240,000 newborns across the world succumb to congenital disorders within 28 days of birth annually, and 3%-6% of infants globally are born with serious birth defects each year. Birth defects stand as a primary cause of infant and young child mortality on a global scale, posing an increased risk of lifelong disabilities for those who survive. Furthermore, an estimated 15 million babies worldwide, exceeding 1 in 10 births, are born preterm each year. Complications arising from preterm births constitute the leading cause of death among children under 5 years old. Thus, the adoption of advanced Neonatal and fetal Monitoring Equipment has emerged as a crucial strategy in contemporary healthcare to combat stillbirths, pre-term births, and birth defects. These monitoring technologies play a pivotal role in enhancing prenatal and neonatal care, offering healthcare providers valuable insights and early detection capabilities to mitigate risks and improve outcomes for both mothers and newborns.
Reducing Stillbirths and Pre-term Births: One of the primary motivations behind the increasing adoption of neonatal and fetal monitoring equipment is the urgent need to address stillbirths and pre-term births.
- Fetal Monitoring Devices: Fetal monitoring devices, such as electronic fetal monitors, enable continuous tracking of fetal heart rates and uterine contractions during pregnancy and labor. This real-time monitoring allows healthcare professionals to detect signs of distress or abnormalities early on, facilitating timely interventions to reduce the risk of stillbirths and pre-term deliveries.
- AI-Guided Assessment of Gestational Age and Fetal Development: A better estimate of gestational age can reduce the number of induced labors and, as a result, help improve the birthing experience for many women. Appropriate targeting of maternal and neonatal care can also help to prevent complications of prematurity.
Early Detection of Birth Defects: Neonatal and fetal monitoring equipment goes beyond basic vital sign monitoring.
- Advanced Imaging Technology: Advanced imaging technologies, such as ultrasound and magnetic resonance imaging (MRI), offer detailed insights into fetal development, helping identify potential birth defects. Early detection allows healthcare teams to develop appropriate management plans, offer counseling to parents, and, in some cases, plan for interventions that can improve the quality of life for newborns with congenital anomalies.
- Blood Test: Tests used to diagnose birth defects may include amniocentesis (also called amnio). This test takes some amniotic fluid from around the baby in the uterus (womb) to check for birth defects and genetic conditions in the baby. This test is usually done at 15 to 20 weeks of pregnancy.
Fill out the "Quick Inquiry Form" to request a sample copy
The Requirement for Neonatal and Fetal Care Equipment in Neonatal Intensive Care Unit (NICU) Propels Market Growth
The initial 28 days of an infant's life, known as the neonatal or newborn period, pose the highest risk of mortality. Neonatal Intensive Care Units (NICUs) are specialized units designed to care for critically ill newborns and can be challenging environments for infants, parents, and caregivers. Nevertheless, advancements in neonatal care have markedly enhanced outcomes for high-risk newborns, propelled by technological innovations specifically tailored for NICUs. Technologies such as respiratory function monitors, video laryngoscopy, and artificial intelligence are becoming increasingly indispensable in NICUs, playing a crucial role in monitoring and providing essential information. The integration of cutting-edge technologies and a focus on patient-centric care will likely propel further innovations in neonatal and fetal care equipment, contributing to the advancement of neonatal healthcare globally. For instance,
- In June 2023, Dräger announced they have redefined open care with the FDA-cleared Babyroo TN300, a new open warmer that offers supportive lung protection and temperature stability from the moment of birth through discharge home
- In September 2022, InnAccel Technologies and the Centre for Cellular and Molecular Platforms (C-CAMP) announced that they have joined hands with the SAMRIDH Healthcare Blended Finance Facility to strengthen respiratory support for newborns and pediatric populations in Assam with an indigenous life-saving technology to reduce neonatal mortality due to respiratory distress syndrome (RDS) in the state
Neonatal and Fetal Care Equipment Market: Key Constraints/ Challenges
The growth of the neonatal and fetal (Labor & Delivery) care equipment market is likely to face impediments due to several factors, including the elevated costs linked to such equipment, a scarcity of specialized neonatal intensive care units (NICUs) in various regions, the challenge of meeting regulatory standards for medical devices, a deficiency in trained healthcare personnel, and the difficulty in ensuring proper sterilization to prevent healthcare-associated infections in neonatal units, particularly in resource-limited settings.
North America Expected to Continue to Hold a Major Share in the Neonatal and Fetal (Labor & Delivery) Care Equipment Market
From a geographical perspective, North America holds a major market share of the Neonatal and Fetal (Labor & Delivery) Care Equipment market. This can be mainly attributed to the high premature birth rate, a significant incidence of neonatal abstinence syndrome (NAS), an increase in maternal mortality rate, lifestyle challenges (such as obesity, smoking, diabetes, and poor nutrition), and the availability of well-equipped NICU centers in the region. However, the Asia-Pacific region is expected to witness strong growth in the coming years due to the increasing prevalence of hospital-acquired infections in newborn babies, rising awareness among the population for the improvement of fetal care and neonatal care, and huge patient populations of preterm & low weight birth babies in the region.
Neonatal and Fetal Care Equipment Market: Competitive Landscape
Some of the key players operating in the market are Atom Medical Corporation, Becton Dickinson, Cardinal Health, Covidien, Dragerwerk AG & Co. Kgaa, Fisher & Paykel Healthcare Limited, GE Healthcare, Inspiration Healthcare Group Plc, Koninklijke, Philips N.V., Masimo, Medtronic, Natus Medical Incorporated, Phoenix Medical Systems, among others.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting organic and inorganic growth strategies such as launching new products, acquiring related firms, and entering into mergers & collaborations to garner higher market share. For instance,
- In November 2023, Royal Philips, a global leader in health technology, announced that they had received a second round of funding from the Bill & Melinda Gates Foundation to accelerate the global adoption of AI algorithms on Philips Lumify Handheld Ultrasound to expand access to maternal health
- In July 2023, Dräger announced that they have received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the Evita V600, V800, and Babylog VN800, its newest devices for mechanical ventilation of adults to premature babies.
- In February 2022, Royal Philips announced it had expanded its ultrasound offerings by enhancing its handheld point-of-care ultrasound, Lumify. The upgrade includes pulse wave Doppler, allowing clinicians to help in the early assessment of gestational age and the identification of high-risk pregnancies.
The Neonatal and Fetal (Labor & Delivery) Care Equipment market is a complex and rapidly evolving industry that is expected to gain further momentum in the coming years due to the availability of a wide variety of devices and technologies, a strong emphasis on developing new products, and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
- What is the market size and forecast for the Neonatal and Fetal (Labor and delivery) Care Equipment Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Neonatal and Fetal (Labor and delivery) Care Equipment Market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the market?
- What is the investment landscape?
- Which region has the highest share in the market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the market?
- What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Industry Speaks
- Market Dynamics
- COVID-19 Impact on the Neonatal & Fetal (Labor & Delivery) Care Equipment Market
- Neonatal & Fetal (Labor & Delivery) Care Equipment Market- Size & Forecast (2021-2028), By Product
- Neonatal Care Equipment
- Incubators
- Respiratory Assistance and Monitoring Devices
- Phototherapy Equipment
- Neonatal Monitoring Devices
- Other Neonatal Care Equipment
- Fetal Care Equipment
- Fetal Dopplers
- Fetal Magnetic Resonance Imaging (MRI) Devices
- Ultrasound Devices
- Fetal Pulse Oximeters
- Other Fetal Care Equipment
- Neonatal Care Equipment
- Neonatal & Fetal (Labor & Delivery) Care Equipment Market- Size & Forecast (2021-2028), By End-User
- Hospitals & Surgical Centers
- Clinics
- Others
- Neonatal & Fetal (Labor & Delivery) Care Equipment Market- Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Medtronic
- Atom Medical Corporation
- Becton Dickinson
- Cardinal Health
- Covidien
- Dragerwerk AG & Co. Kgaa
- Fisher & Paykel Healthcare Limited
- GE Healthcare
- Inspiration Healthcare Group Plc
- Koninklijke Philips N.V.
- Masimo
- Natus Medical Incorporated
- Phoneix Medical Systems
- Other Prominent Players
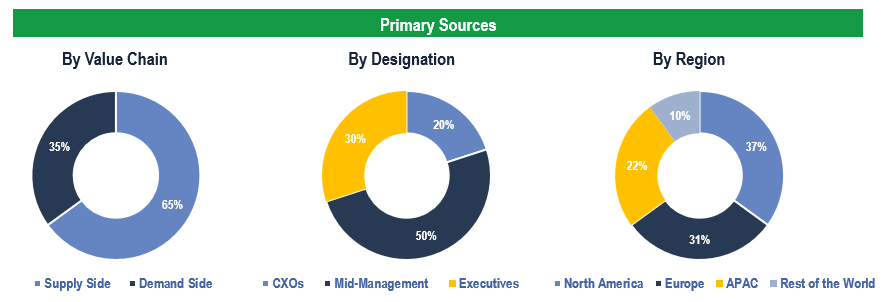
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
- Demand Side Stakeholders: Hospitals & Surgical Centres, and Clinics, Among Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & and internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.