Pharmacovigilance Market Size, Share, Growth, Trends, Supply Chain and Forecast, 2024-2029
The Global Pharmacovigilance (PV) Market is expected to witness a growth rate of 8-9% in the next five years. Increasing incidence of adverse drug reactions (ADRs) and drug withdrawals, growing pharmaceutical and biotech industries, increasingly stringent regulatory mandates and complex compliance requirements throughout the drug lifecycle, growing levels of drug consumption with increasing complexity of drugs being developed, increased outsourcing of PV services by pharmaceutical companies, and the growing adoption of advanced technologies in PV processes are some of the key factors driving pharmacovigilance market growth. However, the high costs of pharmacovigilance activities, the complexity and variability of regulatory requirements across different geographies, concerns regarding data privacy, and limited awareness and expertise in pharmacovigilance in some geographies are likely to hinder the market growth. To learn more about the research report, download a sample report.
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine/vaccine related problem. It involves collecting, monitoring, researching, assessing and evaluating information from healthcare providers and patients on the adverse effects of medications, biological products, vaccines, medical devices, and complementary medicines. The main aim is to identify the earliest possible PV signals and prevent harm to patients. PV plays a key role in the healthcare system through assessment, monitoring and discovery of interactions amongst drugs and their effects in humans.
Increasing Incidence of Adverse Drug Reactions (ADRs) and Drug Withdrawals to Drive Market Growth
The increasing incidence of ADRs and subsequent drug withdrawals is significantly driving the growth of the pharmacovigilance market. ADRs, defined as noxious and unintended responses to medications, pose substantial healthcare challenges, leading to increased hospitalizations and healthcare costs. In 2022 alone, over 1.25 million serious ADRs were reported in the US (Source: NCBI), highlighting the urgent need for effective monitoring systems. As healthcare systems grapple with the complexities of modern therapeutics and an aging population, the demand for robust pharmacovigilance practices intensifies. This includes better reporting mechanisms and risk management strategies to prevent ADRs, thereby fostering a market that prioritizes safety and compliance in drug administration and monitoring. Additionally, with the rise in the number of ADRs, regulatory bodies are enforcing stricter safety standards, compelling pharmaceutical companies to invest in robust pharmacovigilance systems to monitor and manage drug safety. The growing frequency of drug withdrawals due to safety concerns further underscores the need for comprehensive PV practices throughout the drug lifecycle. This heightened focus on drug safety increases the demand for pharmacovigilance services, driving market expansion as companies strive to comply with regulatory requirements, protect patient safety, and avoid costly drug recalls.
“Adverse drug reactions remain a challenge in modern healthcare. With the added complexities of an aging population and increasing multimorbidity, ADRs contribute to a substantial number of unscheduled hospital admissions, indicating a pressing need for robust pharmacovigilance systems to monitor and manage these reactions effectively."- Consultant Physician, A Leading Hospital Group, United States
Increasingly Stringent Regulatory Mandates and Complex Compliance Requirements Throughout the Drug Lifecycle to Fuel Market Growth
The pharmaceutical industry is facing increasingly stringent regulatory mandates and complex compliance requirements throughout the drug lifecycle, which is driving the growth of the pharmacovigilance market. Regulators are focused on ensuring drug safety, efficacy, and quality, with a range of activities spanning the entire drug lifecycle. Regulatory agencies like the FDA, EMA, and other global bodies have heightened their focus on drug safety, requiring pharmaceutical companies to adhere to rigorous PV standards from clinical trials through post-market surveillance. These regulations are designed to ensure that drugs are both safe and effective, and any ADRs are promptly identified and managed. As these regulations become more complex, the burden on pharmaceutical companies to maintain compliance grows. Companies must implement comprehensive PV systems that can handle vast amounts of data, detect safety signals, and report adverse events accurately and timely. Non-compliance can result in severe consequences, including drug recalls, fines, and damage to a company's reputation. This regulatory pressure drives companies to invest heavily in robust PV practices, including advanced technologies, to meet these stringent requirements. Additionally, as new drugs, biologics, and personalized medicines enter the market, the complexity of monitoring their safety increases, further necessitating sophisticated PV systems. The need to comply with these evolving regulations and ensure continuous monitoring across global markets is propelling the growth of the pharmacovigilance market.
To learn more about this report, download the PDF brochure
Continued Trend of Outsourcing of PV Services by Pharmaceutical Companies
The increased outsourcing of PV services by pharmaceutical companies is significantly driving the growth of the pharmacovigilance market. This trend is largely motivated by the need for cost efficiency, regulatory compliance, and access to specialized expertise. Pharmaceutical companies face escalating costs associated with maintaining in-house PV departments, which include hiring and training skilled personnel. By outsourcing these functions to specialized service providers, companies can convert fixed costs into variable ones, aligning expenses more closely with workload demands. This flexibility allows firms to focus on their core competencies, such as research and development, while ensuring that drug safety monitoring is handled by experts well-versed in the latest regulatory requirements and best practices. Moreover, the complexity of regulatory frameworks and the growing volume of data necessitate advanced technological solutions. Outsourcing partners often leverage cutting-edge technologies like artificial intelligence and machine learning to enhance data processing and signal detection, improving overall efficiency and compliance. Additionally, the globalization of clinical trials and the expansion of pharmaceutical markets into emerging regions have increased the demand for localized PV expertise, which outsourcing providers can offer. By outsourcing PV services, pharmaceutical companies can ensure continuous monitoring and reporting of drug safety, ultimately leading to improved patient outcomes and minimizing the risk of costly drug recalls or regulatory penalties. This trend is expected to continue fuelling the growth of the pharmacovigilance market.
North America Expected to be a Major Growth Engine in the Pharmacovigilance Market
North America is expected to be a major growth engine in the pharmacovigilance market, driven by the region's advanced healthcare infrastructure, coupled with the presence of major pharmaceutical companies, creates a strong demand for comprehensive pharmacovigilance services. The US, in particular, plays a pivotal role due to stringent regulatory requirements enforced by the FDA, which mandate rigorous drug safety monitoring throughout the drug lifecycle. The U.S. has one of the most advanced pharmacovigilance systems globally, necessitating comprehensive drug safety monitoring and reporting. The high volume of clinical trials and the introduction of new drugs further drive the demand for sophisticated pharmacovigilance services. Additionally, the high incidence of ADRs and the subsequent focus on patient safety further fuel the need for robust pharmacovigilance systems. The adoption of advanced technologies like AI and big data analytics in pharmacovigilance processes is also more prevalent in North America, enhancing the efficiency and accuracy of safety monitoring, thus driving market growth in the region. The Growth in APAC is driven by the expanding pharmaceutical industry, increased regulatory scrutiny, and rising patient safety concerns. Additionally, the region's rapid adoption of advanced technologies and growing clinical trials further fuels market expansion.
To learn more about this report, download the PDF brochure
Product Type Segment Analysis
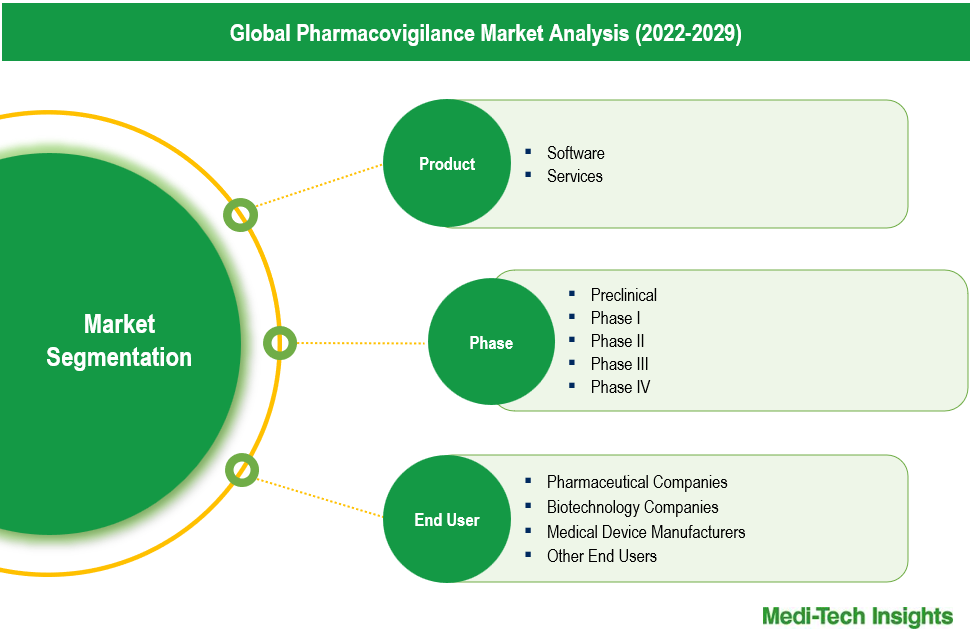
The pharmacovigilance market comprises product types like: Software and Services. the services segment is the largest, driven by the increasing outsourcing of pharmacovigilance activities to specialized service providers. Pharmaceutical companies prefer outsourcing PV services, including adverse event reporting, risk management, and regulatory compliance, to reduce operational costs and focus on core activities like drug development. This trend has made the services segment dominant in the market. Growth in the software segment driven by the rapid adoption of advanced technologies in pharmacovigilance processes. Software solutions enable more efficient data management, real-time monitoring, and automated reporting, which are increasingly vital as the volume of safety data grows. The demand for robust and scalable pharmacovigilance software is rising, particularly as regulatory requirements become more stringent, making this segment the fastest expanding in the market.
End user Type Segment Analysis
The pharmacovigilance market can be analyzed based on end user types, such as Pharmaceutical Companies, Biotechnology Companies, Medical Device Manufacturers, and Other End Users. Pharmaceutical companies are the largest end-user segment of the pharmacovigilance market. The large share of this segment is attributed to high demand for these tools & related services among big pharma, huge investments in clinical development & manufacturing, need to comply with changing regulatory guidelines & submission standards, and growing complexity related to drug safety regulations. Growth in the biotechnology companies segment is driven by the rapid advancement and commercialization of biologics, gene therapies, and personalized medicines have increased the complexity of drug safety monitoring. As these companies bring innovative and often more complex therapies to market, the need for specialized pharmacovigilance services grows. Additionally, the expansion of clinical trials for biotechnology products, coupled with stringent regulatory requirements, is driving the accelerated growth of pharmacovigilance activities within this segment.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner market share. For instance,
- In July 2024, Oracle introduced new AI-enhanced capabilities in its Argus and Safety One Intake solutions to help life science organizations manage increasing adverse event workloads and meet evolving global regulatory requirements. These updates automate safety case processing, enhance data privacy and reporting, and improve compliance with country-specific regulations, optimizing efficiency for pharmaceutical, medical device companies, and CROs
- In April 2024, Qinecsa Solutions acquired Danish based Insife ApS to enhance its position as one of the leading providers of digital pharmacovigilance solutions. The acquisition combines Qinecsa’s expertise with Insife’s HALOPV platform, strengthening their global offerings in drug safety data management and pharmacovigilance technologies
- In December 2023, Thermo Fisher Scientific launched CorEvidence, a cloud-based data lake platform designed to optimize pharmacovigilance case processing and safety data management, enhancing its CorEvitas clinical research registries. The platform streamlines the management of adverse events and supports regulatory requirements for long-term post-authorization safety studies
- In October 2023, IQVIA entered into a strategic collaboration with argenx to enhance treatment for rare autoimmune diseases through innovative, integrated pharmacovigilance (PV) safety services and solutions. This partnership will support argenx in efficiently integrating safety data, streamlining adverse event reporting, and scaling its PV operations as the company grows
- In November 2022, Clinigen acquired Drug Safety Navigator Inc., a US based specialist pharmacovigilance service provider, to enhance its pharma services offering for pharmaceutical and biotech clients. This acquisition expands Clinigen's capabilities in adverse event reporting, medical monitoring, and global pharmacovigilance services across the medical product lifecycle
The pharmacovigilance market is expected to gain further momentum in the coming years due to technological advancements, rising R&D investments, and aggressive organic and inorganic growth strategies followed by the players.
Competitive Landscape Analysis: Pharmacovigilance Market
The global Pharmacovigilance market is marked by the presence of established market players such as Accenture plc, ArisGlobal, Certara, ClinChoice, Clinigen Limited, Cognizant, Deloitte, ICON plc, IQVIA Inc, Oracle, Oviya MedSafe, Parexel, Qinecsa Solutions, Syneos Health, TAKE Solutions, Thermo Fisher Scientific, United BioSource LLC, Veeva Systems, and Wipro among others.
Get a sample report for competitive landscape analysis
Future Outlook of the Pharmacovigilance Market
The global pharmacovigilance market is expected to gain further momentum in the coming years due to the constantly rising investment in R&D by healthcare companies, increasing partnerships and collaborations between market players, and increased externalization of clinical trial studies by large pharmaceutical and biopharmaceutical companies. These factors collectively contribute to the growth and evolution of the pharmacovigilance market.
Pharmacovigilance Market Report Scope
| Report Scope | Details |
| Base Year Considered | 2023 |
| Historical Data | 2022 - 2023 |
| Forecast Period | 2024 - 2029 |
| Growth Rate | 8-9% |
| Segment Scope | Product, Application, End User |
| Regional Scope |
|
| Key Companies Mapped | Accenture plc, ArisGlobal, Certara, ClinChoice, Clinigen Limited, Cognizant, Deloitte, ICON plc, IQVIA Inc, Oracle, Oviya MedSafe, Parexel, Qinecsa Solutions, Syneos Health, TAKE Solutions, Thermo Fisher Scientific, United BioSource LLC, Veeva Systems, and Wipro among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Pharmacovigilance Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Pharmacovigilance Market?
-
How has COVID-19 impacted the Global Pharmacovigilance Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical and Biotechnology Companies, Medical Device Companies and Other End Users
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.