Global Point of Care Molecular Diagnostics Market Size & Trends Report Segmented by Technology (PCR, Genetic Sequencing), Application (Infectious Diseases, Cancer, Hematology), End-user (Hospitals, Diagnostic Centers, Home Care) & Regional Forecast to 2030
The point of care molecular diagnostics market is experiencing steady growth, with a CAGR of ~6% driven by increasing demand for rapid and decentralized diagnostic solutions. Key factors propelling market expansion include the rising prevalence of infectious diseases, growing adoption in resource-limited settings, and advancements in miniaturized diagnostic technologies. However, challenges such as regulatory complexities and high initial costs for POC molecular platforms may hinder widespread adoption. To learn more about the research report, download a sample report.
Report Overview
Point of care molecular diagnostics refers to advanced diagnostic testing conducted at or near the patient site, enabling rapid detection of pathogens and genetic markers. Unlike traditional lab-based molecular diagnostics, POC molecular testing integrates sample preparation, amplification, and detection into a compact system, reducing turnaround time from hours to minutes. These diagnostics leverage technologies like PCR and isothermal amplification, ensuring high sensitivity and specificity in detecting infectious diseases, genetic conditions, and oncology markers. Their significance lies in improving early diagnosis, guiding timely treatment, and enhancing healthcare accessibility, particularly in remote or under-resourced areas.
To learn more about the research report, download a sample report.
Widespread infectious disease burden accelerating market growth
The increasing prevalence of infectious diseases is a primary factor fueling the expansion of the POC Molecular Diagnostics market. The growing global burden of respiratory infections, sexually transmitted diseases, and emerging viral outbreaks necessitates rapid and decentralized diagnostic solutions. Conventional laboratory-based molecular diagnostics, while highly accurate, often require centralized facilities and lengthy turnaround times, delaying treatment decisions. POC molecular platforms bridge this gap by delivering near-instant results at the patient’s location, facilitating prompt clinical intervention. Additionally, healthcare systems in low- and middle-income countries benefit from these rapid diagnostics, as they reduce the dependency on specialized infrastructure and skilled personnel. The COVID-19 pandemic further underscored the importance of fast, accurate molecular testing at the point of care, accelerating investment in portable diagnostic devices. As healthcare providers prioritize early disease detection and containment strategies, the demand for POC molecular diagnostics continues to surge, reshaping infectious disease management worldwide.
To learn more about the research report, download a sample report.
Microfluidics revolutionizing POC molecular testing
Microfluidic-based POC molecular diagnostics represent a significant innovation enhancing the market’s effectiveness and adoption. Microfluidics enables the miniaturization and automation of molecular assays, integrating multiple testing steps—sample extraction, amplification, and detection—into a single, compact chip. These systems require minimal sample volumes, reducing reagent costs while maintaining high analytical sensitivity. Additionally, microfluidic platforms improve assay reproducibility and reliability, addressing variability concerns in traditional manual methods. The portability of microfluidic-based devices makes them ideal for field settings, rural clinics, and emergency response situations, where timely diagnostics are critical. Moreover, advancements in lab-on-a-chip technology have streamlined complex workflows, enabling non-specialists to conduct sophisticated molecular testing with minimal training. As the technology matures, microfluidic POC diagnostics are expected to drive broader adoption, particularly in infectious disease surveillance, antimicrobial resistance monitoring, and personalized medicine applications.
Competitive Landscape Analysis
The global point of care molecular diagnostics market is marked by the presence of established and emerging market players such as Abbott; F. Hoffmann-La Roche AG; QIAGEN; Danaher; Bio-Rad Laboratories, Inc.; bioMérieux; Agilent Technologies, Inc.; Nova Biomedical; Nipro Diagnostics and Thermo Fisher Scientific among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Report Scope
|
Report Scope |
Details |
|
Base Year Considered |
2024 |
|
Historical Data |
2023 - 2024 |
|
Forecast Period |
2025 – 2030 |
|
Growth Rate |
~6% |
|
Market Drivers |
|
|
Attractive Opportunities |
|
|
Segment Scope |
Technology, Application, and End-user |
|
Regional Scope |
|
|
Key Companies Mapped |
Abbott; F. Hoffmann-La Roche AG; QIAGEN; Danaher; Bio-Rad Laboratories, Inc.; bioMérieux; Agilent Technologies, Inc.; Nova Biomedical; Nipro Diagnostics and Thermo Fisher Scientific among others |
|
Report Highlights |
Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Market Segmentation
This report by Medi-Tech Insights provides the size of the point of care molecular diagnostics market at the regional- and country-level from 2023 to 2030. The report further segments the market based on technology, application and end-user.
- Market Size & Forecast (2023-2030), By Technology, USD Million
- PCR-based
- Genetic Sequencing-based
- Microarray-based
- Others
- Market Size & Forecast (2023-2030), By Application, USD Million
- Infectious Diseases
- Cancer
- Endocrinology
- Hematology
- Others
- Market Size & Forecast (2023-2030), By End-user, USD Million
- Hospitals
- Diagnostic Laboratories
- Home Care Settings
- Others
- Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
- North America
Key Strategic Questions Addressed
- What is the market size & forecast of the point of care molecular diagnostics market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the point of care molecular diagnostics market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2025-2026)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- Point of Care Molecular Diagnostics Market Snapshot (2025-2030)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Rising prevalence of infectious diseases and antimicrobial resistance
- Growing demand for rapid, decentralized diagnostic solutions
- Increased adoption of POC testing in resource-limited settings
- Regulatory support for faster diagnostic approvals
- Integration of multiplex testing for broader disease detection
- Restraints
- High costs associated with advanced molecular POC platforms
- Technical limitations affecting sensitivity and specificity in some tests
- Limited awareness and adoption in developing regions
- Opportunities
- Rising use of CRISPR-based molecular diagnostics
- Growth of multiplexed molecular assays for simultaneous detection
- Expansion of POC molecular diagnostics in home care and telemedicine
- Adoption of fully automated, cartridge-based POC platforms
- Key Market Trends
- Advancements in microfluidic and lab-on-a-chip technologies
- Growing potential in veterinary and agricultural disease testing
- Drivers
- Market Dynamics
-
- Unmet Market Needs
- Industry Speaks
- Global Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Technology, USD Million
- Introduction
- PCR-based
- Genetic Sequencing-based
- Microarray-based
- Others
- Global Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Application, USD Million
- Introduction
- Infectious Diseases
- Cancer
- Endocrinology
- Hematology
- Others
- Global Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By End-user, USD Million
- Introduction
- Hospitals
- Diagnostic Laboratories
- Home Care Settings
- Others
- Global Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Region, USD Million
- Introduction
- North America Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- US
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Canada
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- US
- Europe Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- UK
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Germany
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- France
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Italy
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Spain
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Europe
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- UK
- Asia Pacific (APAC) Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), By Country, USD Million
- China
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Japan
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- India
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- China
- Latin America (LATAM) Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Middle East & Africa (MEA) Point of Care Molecular Diagnostics Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Technology (USD Million)
- Market Size & Forecast, By Application (USD Million)
- Market Size & Forecast, By End-user (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2024)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2025)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles* (Business Overview, Financial Performance**, Products Offered, Recent Developments)
- Abbott
- Hoffmann-La Roche AG
- QIAGEN
- Danaher
- Bio-Rad Laboratories, Inc.
- bioMérieux
- Agilent Technologies, Inc.
- Nova Biomedical
- Nipro Diagnostics
- Thermo Fisher Scientific
- Other Prominent Players
Note: *Indicative list
**For listed companies
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
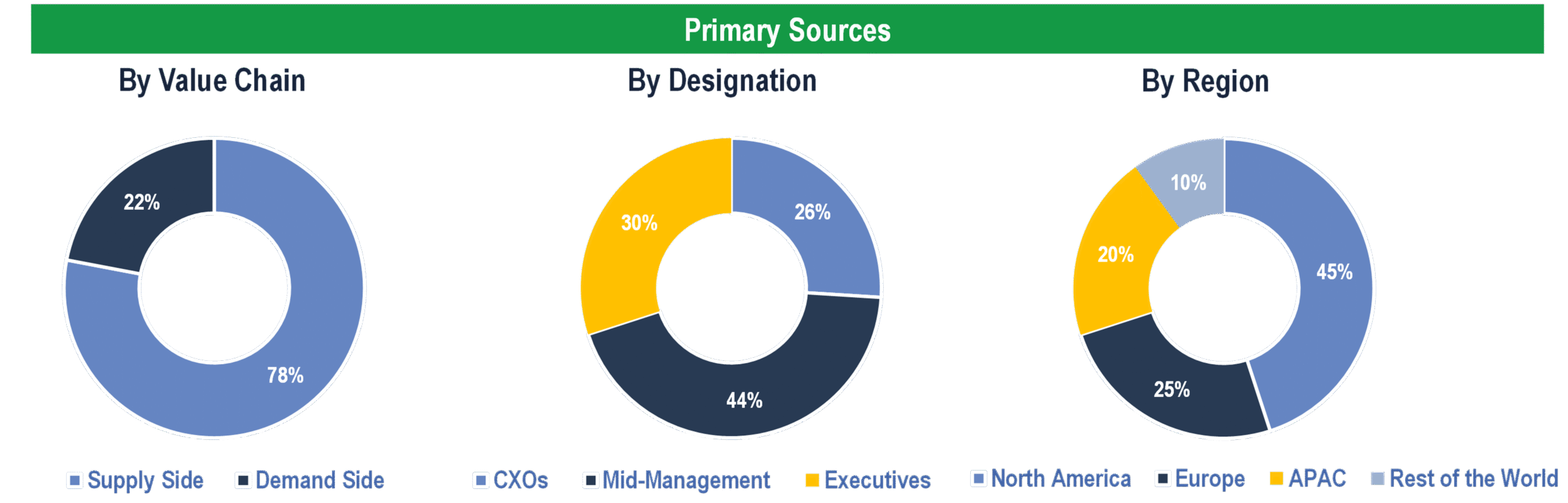
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders from Hospitals, Diagnostic Laboratories, Home Care Settings, and Others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data