Psoriasis Treatment Market Size, Share, Growth & Analysis Report 2026
The Global Psoriasis Treatment Market is expected to grow at a rate of 8.5% by 2026. The growing prevalence of psoriasis, increased demand for psoriasis medicines in emerging economies, growing approval of new biologics drugs for psoriasis treatment, increased activity for psoriasis research, a strong pipeline of drugs, and increased usage of combined therapies for psoriasis treatment are some of the key factors driving the psoriasis treatment market growth.
Psoriasis is a complex, chronic, multifactorial, inflammatory disease that involves hyperproliferation of the keratinocytes in the epidermis, with an increase in the epidermal cell turnover rate. There are mainly five types of psoriasis, such as plaque, flexural, guttate, nail, and psoriatic arthritis.
The disease most commonly manifests on the skin of the elbows, knees, scalp, lumbosacral areas, intergluteal clefts, and glans penis. In up to 30% of patients, the joints are also affected. Psoriasis treatments are based on surface areas of involvement, body sites affected, the presence or absence of arthritis, and the thickness of the plaques and scale. Currently, topical, systemic therapies, and phototherapy are available for treatment. In practice, a combination of these methods is often used.
Growing Adoption of New Biologics Drugs for the Treatment of Plaque Psoriasis Drives the Psoriasis Treatment Market
Biologics drugs launched a new era of psoriasis treatment. These drugs are made from substances found in living things and target a specific part of the immune system. The drugs block certain cells or proteins that play a role in psoriasis thus keeping them from going into overdrive. Recently, several biological drugs have been approved by the US Food and Drug Administration (FDA) to treat plaque psoriasis. For instance,
- In May 2022, Dermavant Sciences received United States Food and Drug Administration (FDA) approval for VTAMA (tapinarof) cream, 1%, an aryl hydrocarbon receptor agonist, indicated for the topical treatment of plaque psoriasis in adults. The approval makes VTAMA cream the first and only FDA-approved steroid-free topical medication in its class.
- In August 2021, Novartis secured a new approval in China for Cosentyx® (secukinumab) for the treatment of pediatric, moderate to severe plaque psoriasis.
- In February 2021, Amgen announced the submission of a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for Otezla (apremilast) for the treatment of adults with mild-to-moderate plaque psoriasis.
Increase in Usage of Interleukins (IL) Inhibitors in Psoriasis Treatment Fuels the Market Demand
Interleukin inhibitors are immunosuppressive agents that inhibit the action of interleukins. They are used to treat various inflammatory and autoimmune diseases. Earlier, pharmacological treatment consisted of non-biological products such as topical corticosteroids, vitamin D analogues, keratolytics, and calcineurin inhibitors. However, the availability of biologics targeting specific immunologic pathways implicated in the pathogenesis of the disease, such as the tissue necrosis factor (TNF) inhibitors and the interleukin (IL) inhibitors resulted in significantly improved treatment for psoriasis.
The preference for IL inhibitors over other classes of psoriasis drugs includes several factors such as improved safety and efficacy, more targeted mechanism of action, fewer warnings & side effects, faster onset of effect, and more durable responses. Recently, several drugs have been approved by the U.S. Food and Drug Administration (FDA) for treatment. For instance,
- In January 2022, AbbVie received U.S. Food and Drug Administration (FDA) approval for SKYRIZI® (risankizumab-rzaa) for the treatment of adults with active psoriatic arthritis (PsA), a systemic inflammatory disease that affects the skin and joints and impacts approximately 30 percent of patients with psoriasis. SKYRIZI is an interleukin-23 (IL-23) inhibitor that selectively blocks IL-23 by binding to its p19 subunit.
- In December 2021, Novartis received the US Food and Drug Administration (FDA) approval for Cosentyx® (secukinumab) for the treatment of active enthesitis-related arthritis (ERA) in patients four years and older, and active psoriatic arthritis (PsA) in patients two years and older.
Growing Usage of Combination Therapies for Psoriasis Treatment Boost the Psoriasis Treatment Market
Combination therapy is the most common way to treat psoriasis. It involves the combination of two drugs to achieve improved therapeutic results for patients who inadequately respond to a single drug. Moreover, combination therapy may reduce safety concerns and cumulative toxicity, as a lower dose of two single agents may offer a better safety and efficacy profile when used in combination. The wide range of combination therapies prescribed may reflect increased attention to individualization of treatment to match patients’ diverse preferences. Currently approved topical treatments include prescription medications such as corticosteroids (most commonly used alone or in combination therapy), vitamin D derivatives, vitamin A derivatives (tazarotene), and anthralin. The most commonly used combinations are topical steroid plus other topical, multiple topical steroids, topical steroid plus vitamin D analogue, and topical steroid plus systemic treatment. The growing adoption of combination therapies is likely to fuel the psoriasis treatment market in the coming years.
Key Constraints/Challenges: Psoriasis Treatment Market
The treatment used for psoriasis can be expensive, life-long, and often not financed by universal health coverage schemes. Lack of standard tools for the diagnosis and treatment, stringent regulatory policies for the approval process and new drug development, and adverse side effects associated with the existing medications are some of the key factors that are likely to hamper the psoriasis treatment market in the upcoming years.
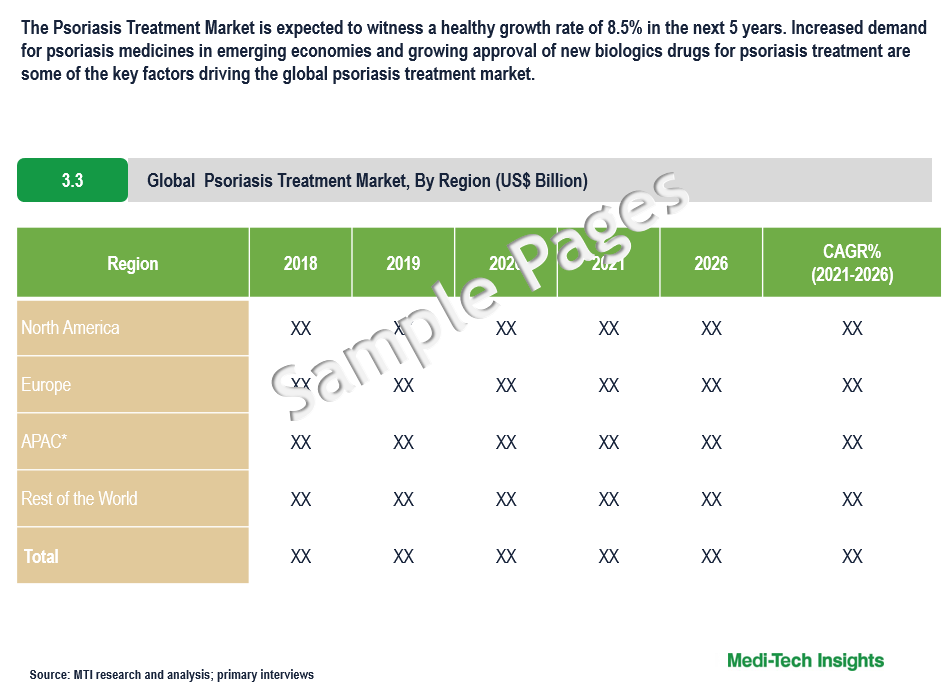
North America Expected to Continue to Hold a Major Share in the Psoriasis Treatment Market
From a geographical perspective, North America holds a major market share of the psoriasis treatment market. This can be mainly attributed to the growing prevalence of plaque psoriasis & psoriatic arthritis, the rising prescription volume of biological products, and growing awareness regarding the diagnosis and treatment of psoriasis in the region. However, the Asia-Pacific region is expected to witness strong growth in the coming years due to increasing awareness regarding psoriasis, an increase in demand for biological therapies, and the upsurge in research and development activities for psoriasis treatment and pipeline drugs in the region.
Competitive Landscape Analysis: Psoriasis Treatment Market
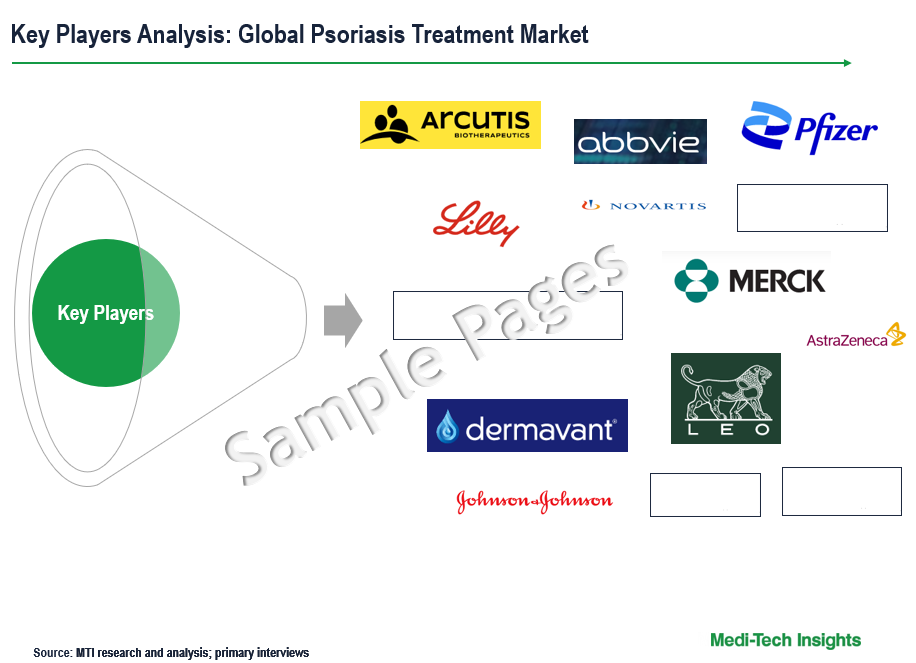
Some of the key players operating in the global psoriasis treatment market include Eli Lilly & Company, Pfizer Inc., Janssen Global Services LLC, Celgene Corporation, Takeda Pharmaceutical Company Limited, Novartis International AG, Amgen Inc., Biogen Inc., Abbvie Inc., AstraZeneca, Boehringer Ingelheim International, Johnson & Johnson Services Inc., Merck & Co. Inc., Sun Pharmaceuticals Industries Ltd., Arcutis Biotherapeutics Inc., UCB S.A., Leo Pharma, Cipla Inc., Rowan Bioceuticals, Glenmark Pharmaceuticals, Dr. Reddy`s Laboratories, and Evelo Biosciences Inc, among others.
Increasing Emphasis on Developing New Biological Products
Players operating in this market are adopting organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner market share. For instance,
- In July 2022, Arcutis Biotherapeutics, Inc. received the United States Food and Drug Administration (FDA) approval for the New Drug Application (NDA) for ZORYVE (roflumilast) cream 0.3% for the treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age or older.
- In December 2021, AbbVie received U.S. Food and Drug Administration (FDA) approval for RINVOQ® (upadacitinib; 15 mg, once daily) for the treatment of adults with active psoriatic arthritis (PsA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
- In October 2021, Sun Pharmaceutical Industries Ltd. announced that their ILUMYA injections are now available in Canada for the treatment of moderate-to-severe plaque psoriasis.
The worldwide psoriasis treatment market is a growing market that is expected to gain further momentum in the coming years due to a strong emphasis on developing new biologics products, increased access to psoriasis treatment in developing economies, growing approval for new drugs, and aggressive organic and inorganic growth strategies followed by the market players.
Key Strategic Questions Addressed
- What is the market size & forecast for the psoriasis treatment market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the psoriasis treatment market?
- How has Covid-19 impacted the psoriasis treatment market?
- What are the major growth drivers, restraints/challenges impacting the global market?
- What are the opportunities prevailing in the psoriasis treatment market?
- What is the investment landscape of psoriasis treatment market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the psoriasis treatment market?
- What are the key strategies adopted by players operating in psoriasis treatment market?
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
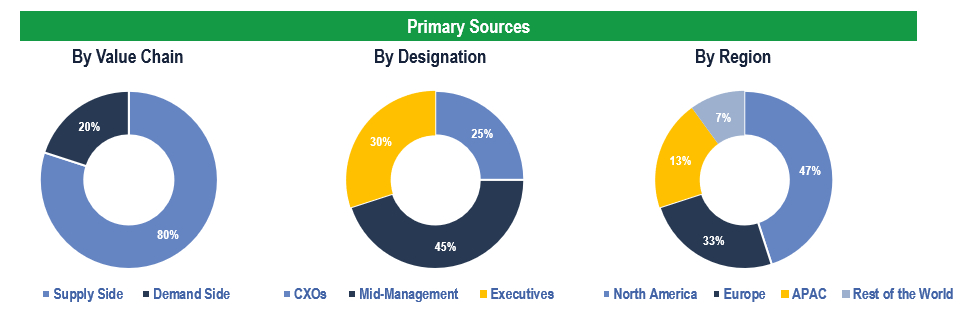
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Pharmacies, Clinics, Retail Pharmacies and Other End Users.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.