Structural Heart Devices Market Size, Share, Growth, Trends & Demands 2026
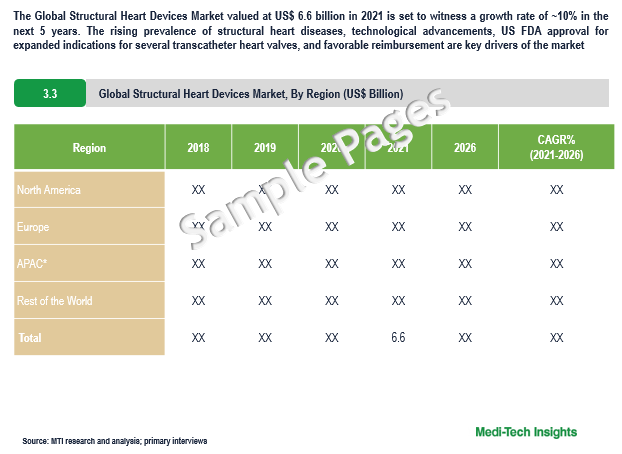
The Global Structural Heart Devices Market valued at $6.6 billion in 2021 is set to witness a growth rate of 10% in the next 5 years. The rising geriatric population & corresponding rise in the prevalence of structural heart disease, technological advancements in structural heart devices, US FDA approval for expanded indications for several transcatheter heart valves, increasing awareness about structural heart diseases, and favorable reimbursements for structural heart procedures in key markets are some of the pivotal factors driving the growth of the structural heart devices market.
Structural heart disease occurs when something is wrong with one’s heart valves, walls, chambers or muscles. It exists from birth or develops as one age. It includes conditions such as congenital heart disease, cardiomyopathy, and heart valve disease. Heart valve devices, occluders & delivery systems, annuloplasty rings, heart access systems, closure devices, introducers, sheaths, balloon catheters, and accessories are some of the devices used for structural heart disease treatment.
APAC to Witness Highest Growth in the Next Five Years
A rising prevalence of cardiovascular diseases, a higher degree of unmet clinical needs, and rising affluence are creating ample opportunities for the structural heart devices market in the APAC region. The growth of the structural heart devices market in the APAC is likely to be driven by China and India. Considering the lucrative prospects of the structural heart devices market in the APAC region, prominent companies have expanded their footprint in the region. For instance,
- In Jan 2022, Medtronic received approval of the CoreValve™ Evolut™ PRO TAVR system from National Medical Products Administration (NMPA) in China. CoreValve™ Evolut™ PRO TAVR system is aimed for the treatment of severe aortic stenosis (AS) for symptomatic patients in China who are at high or extreme risk for open heart surgery.
- In June 2020, Edwards Lifesciences Corporation received Chinese regulatory approval for the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients suffering from severe, symptomatic aortic stenosis (AS) at high risk for or unable to undergo open-heart surgery.
“Aortic stenosis affects more than 5 million patients in China and it is expected to reach more than 7 million by 2030. The clinical demand for treatment of severe aortic stenosis is expected to increase due to the growing aging population in China. Because of the advantages of minimally invasive procedure & quick recovery, transcatheter aortic valve replacement (TAVR) is fast-emerging as a suitable option for several elderly patients in China.” -Academician & Director, Leading Cardiology Hospital, China
Transcatheter Mitral Valve Replacement Systems (TMVR), Tricuspid Valve Repair, and Tricuspid Valve Replacement Systems Likely to Open New Growth Avenues
In the field of structural heart disease, interventional treatment for mitral regurgitation is one of the most challenging areas but showcases huge market potential. Mitral valve regurgitation is the most common type of heart valve disease in which the valve between the left heart chambers does not close completely, allowing blood to leak backward across the valve. Similarly, tricuspid regurgitation is a highly prevalent, but rarely treated disease. It occurs when the valve’s flaps do not close properly. As a result, blood can leak backward into the atrium from the leaky tricuspid valve, causing the heart to pump harder to move blood through the valve. Tricuspid regurgitation affects more than 2 million patients in the United States and represents a large, unmet clinical need.
Citing the lucrative prospects and unmet needs of the market, players operating in the market are launching innovative devices to address the demand. For instance,
- In January 2022, InnovHeart s.r.l., a developer of novel Transcatheter Mitral Valve Replacement (TMVR) systems for the treatment of mitral valve disease raised $55 million in Series C financing to further develop the Trans-septal Saturn Transcatheter Mitral Valve Replacement System.
- In September 2020, U.S. FDA approved an early feasibility study (EFS) of the Intrepid™ Transcatheter Tricuspid Valve Replacement (TTVR) system in patients with severe, symptomatic tricuspid regurgitation. Medtronic also received Breakthrough Device Designation by the FDA for the Intrepid Transcatheter Tricuspid Valve Replacement (TTVR) system.
- In January 2020, Abbott (NYSE: ABT) announced that its Tendyne™ Transcatheter Mitral Valve Implantation (TMVI) system received CE Mark and approval for use in Europe. The system treats mitral regurgitation (MR) in patients requiring a heart valve replacement and provides a safe and effective solution for MR patients who are not candidates for open-heart surgery or transcatheter mitral valve repair.
Technological Advancements Drive the Structural Heart Devices Market
The structural heart devices market is a technology-driven market and is marked by product enhancements/innovations. For instance,
- In September 2021, Abbott received U.S. FDA approval for Portico™ with FlexNav™ transcatheter aortic valve replacement (TAVR) system. The system treats people with symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery.
- In May 2021, Abbott received CE Mark for its latest-generation transcatheter aortic valve implantation (TAVI) system, Navitor™. The minimally invasive device is now available for people in Europe with severe aortic stenosis who are at high or extreme surgical risk.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in Structural Heart Devices Market
The structural heart devices market is marked by the presence of both established and new players. Players operating in the market adopt both organic and inorganic growth strategies such as acquisitions, and new product launches to garner market share. For instance,
- In February 2022, Genesis MedTech Group completed the acquisition of JC Medical (JCM), a structural heart company engaged in the design and development of transcatheter valve replacement products for the minimally invasive treatment of structural heart diseases. The acquisition adds J-Valve, a minimally invasive transcatheter aortic valve replacement (TAVR) device for both aortic regurgitation and stenosis patients, to Genesis product portfolio and significantly improves the Group's ability to better meet the needs of patients, interventional cardiologists, and cardiac surgeons.
- In February 2022, Boston Scientific announced the close of its acquisition of Baylis Medical Company Inc., a company that offers advanced transseptal access solutions as well as guidewires, sheaths and dilators used to support catheter-based left-heart procedures. The acquisition allows Boston Scientific to integrate the Baylis platforms with its existing electrophysiology and structural heart offerings, further strengthening its position in the cardiology market.
- In June 2021, CORCYM, the new independent medical device company announced its entry into the market with state-of-the-art surgical solutions to fight structural heart disease. CORCYM is born from the acquisition of (LivaNova) heart valve business by Gyrus Capital.
The global structural heart devices market is expected to continue to grow in the coming years due to growing cardiovascular cases, growth opportunities in the APAC region, technological advancements in structural heart devices, and favorable reimbursement in key markets.
Competitive Landscape Analysis: Structural Heart Devices Market
The structural heart devices market is marked by the presence of established players such as Edwards Lifesciences, Medtronic, Boston Scientific, Abbott, SMT, TTK Healthcare, Micro Interventional Devices (MID) Lepu Medical, Microport, CORCYM, among others.
Key Strategic Questions Addressed
- What is the market size & forecast of the Structural Heart Devices Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Structural Heart Devices Market?
- What are the key trends defining the structural heart devices market?
- What are the major factors impacting the structural heart devices market?
- What are the opportunities prevailing in the structural heart devices market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the structural heart devices market?
- What are the key strategies adopted by players?
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
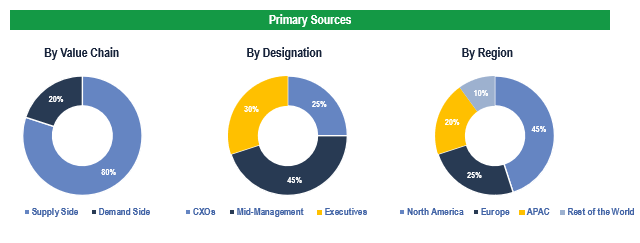
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Ambulatory Surgery Centers, Cardiac Centers, among others.
Breakdown of Primary Interviews
Market Size Estimation
The market size was derived based on extensive secondary research further validated through expert interviews.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with primary interviews and an internal knowledge repository.