Tricuspid Valve Repair Market Size, Industry Growth Analysis, Market Share, Trends, and Forecast 2023 to 2028
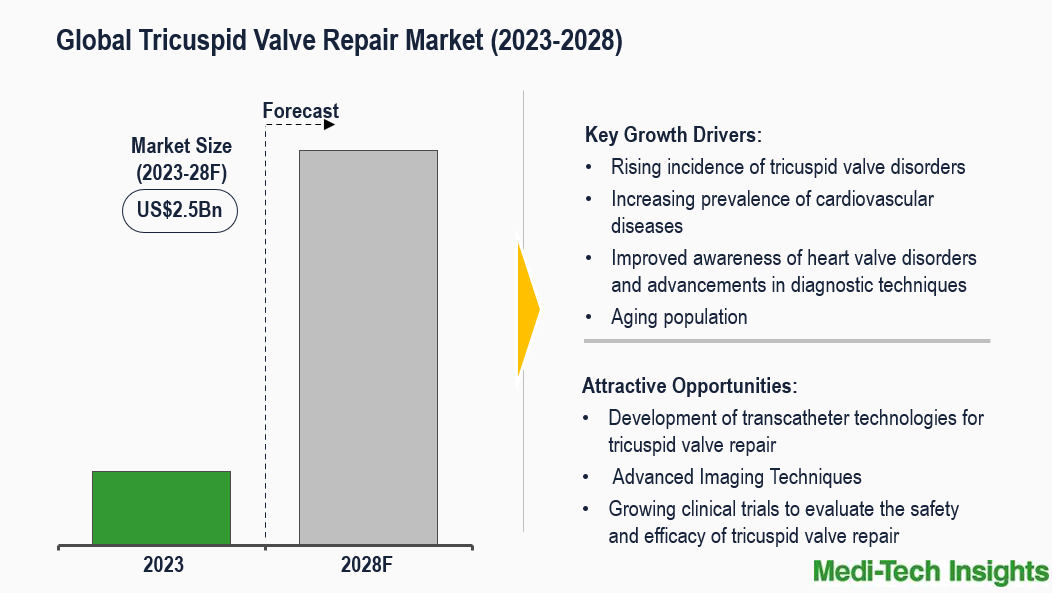
The Global Tricuspid Valve Repair Market is expected to reach a US$2.5 billion market by 2028. Rise in the incidence of tricuspid valve disorders, advancements in medical technology in the field of cardiac interventions, improved awareness of heart valve disorders, a rising geriatric population, growing preference for minimally invasive procedures such as annuloplasty and transcatheter tricuspid valve repair (TTVR), and increase in clinical research and trials activities for developing new tricuspid valve repair technologies are some of the key factors driving the global tricuspid valve repair market. To learn more about the research, fill out a quick inquiry for a sample report.
The tricuspid valve is one of four valves that control blood flow through our heart. It’s on the right side of our heart, between the upper chamber (atrium) and lower chamber (ventricle). It ensures that blood flows in the correct direction from the right atrium down to the right ventricle. The tricuspid valve has three flaps, called leaflets that open and close as blood flows through it. Some people have health conditions that affect the tricuspid valve’s function, called tricuspid valve diseases. The main types are:
Tricuspid Regurgitation: The valve leaks or doesn't close tightly enough, allowing blood to leak backward.
Tricuspid Stenosis: The valve’s leaflets are too stiff, which can restrict blood from flowing.
Tricuspid valve replacement and repair is a medical procedure that treats diseases affecting one of four heart valves. Repairing a patient’s valve rather than replacing it with a prosthetic device is generally preferable. Valve repair usually involves the surgeon modifying the tissue or underlying structures of the mitral or tricuspid valves. Nearly all valve repairs include the placement of an annuloplasty ring or band. This is a cloth-covered device that is implanted around the circumference, or annulus, of the mitral or tricuspid valve. It provides support to the patient’s valve and brings the valve leaflets closer together, potentially reducing leaks across the valve. This procedure can ease symptoms and increase survival in people with tricuspid regurgitation or tricuspid stenosis.
Advancement in Managing Tricuspid Regurgitation Fuels the Demand for Tricuspid Valve Repair Market
Tricuspid regurgitation (TR) is the most common indication requiring tricuspid valve repair. There are an estimated 1.6 million people in the United States with moderate to severe tricuspid regurgitation in contrast to only a few thousand tricuspid valve repair procedures performed annually. The patient population is mostly elderly and suffers from fatigue, limited exercise tolerance, peripheral oedema, and abdominal swelling. Managing these symptoms requires conservative drugs, such as high-dose diuretics, but the clinical effect is not significant. In addition, tricuspid valve surgery has been included in clinical authoritative guidelines, but compared with other valvular diseases, its treatment rate is lower and the mortality rate is higher. Due to significant co-morbidities, less than 1% of patients receive surgical treatment. For most patients, the only option is medical therapy to achieve a temporary reduction in volume overload. Therefore, there is an increasing demand for minimally invasive procedures over traditional open-heart surgeries for treating tricuspid regurgitation. For instance,
- In October 2023, Edwards Lifesciences Corporation announced the company's EVOQUE tricuspid valve replacement system received CE Mark for the transcatheter treatment of eligible patients with tricuspid regurgitation (TR)
- In April 2021, Abbott announced that they had received CE Mark for its next-generation TriClip Transcatheter Tricuspid Valve Repair System, the first-of-its-kind minimally invasive tricuspid heart valve repair device available in Europeto treat tricuspid regurgitation (TR)
Fill out the "Quick Inquiry Form" to request a sample copy
Tricuspid Valve Repair Market: Emerging Trends
Some of the emerging trends in the tricuspid valve repair market are:
Transcatheter Approaches: There are many innovative technologies under investigation for transcatheter tricuspid valve repair targeting leaflet coaptation, annuloplasty, and prosthetic valve deployment. Percutaneous transcatheter tricuspid repair techniques can be broadly divided into the following categories direct suture annuloplasty, direct ring annuloplasty, and coaptation enhancement. For instance,
- In September 2022, Cardiac Implant LLC, announced the successful initial deployment of its Annuloplasty ring with the implementation of a therapeutic adjustment procedure using its innovative Tri-Ring™ percutaneous annuloplasty device
Clip Technologies: Companies like Abbott with their TriClip system have been working on innovative clip technologies. Abbott's TriClip device is a first-of-its-kind, minimally invasive device designed specifically for tricuspid heart valve repair. For instance,
- In May 2023, Abbott announced its late-breaking data that added to the body of clinical evidence supporting the benefits of the TriClip transcatheter edge-to-edge repair (TEER) system in treating patients with leaky tricuspid valves
Clinical Trials: The influx of high investments into clinical trials by manufacturers, and the introduction of novel devices provide numerous growth opportunities for market players. Ongoing research focused on understanding the pathology of tricuspid valve diseases, contributes to the development of targeted and effective treatment strategies.
- In September 2020, Medtronic plc, announced U.S. Food and Drug Administration (FDA) approval of an early feasibility study (EFS) of the Intrepid Transcatheter Tricuspid Valve Replacement (TTVR) system in patients with severe, symptomatic tricuspid regurgitation
Bioprosthetic Valves: An increasing number of patients undergoing tricuspid valve replacement (TVR) have received bio-prostheses due to the great durability in the tricuspid position and to the advantage of freeing patients from anticoagulant medication.
Tricuspid Valve Repair Market: Key Constraints/Challenges
The tricuspid valve repair market faces several challenges and restraints. The costs associated with cardiac surgeries, including tricuspid valve repair procedures can be significant. Tricuspid valve repair or replacement is invasive and presents several risks/complications that include heart blocks, infections, bleeding, prosthetic valve dysfunction, stroke, and death. Tricuspid valve repair often requires specialized skills and expertise. Also, the availability of alternative treatment options or devices for tricuspid valve disorders may impact market growth.
North America Accounts for the Largest Share of the Global Market
North America has emerged as the predominant global market for tricuspid valve repair. The region experiences substantial market growth, driven by stringent favorable regulatory environments for the approval and commercialization of innovative tricuspid valve repair devices, an increase in the prevalence of cardiovascular diseases, including valvular disorders, the growing aging population, and technological advancement contributes significantly to the market growth. The European region is expected to witness lucrative growth during the forecast period. The advancements in healthcare infrastructure, growing awareness of heart valve disorders, the prevalence of cardiovascular diseases, and increasing healthcare expenditure present significant growth opportunities for the tricuspid valve repair market in the region.
Tricuspid Valve Repair Market: Competitive Landscape
The top market players in the global tricuspid valve repair market include Edwards Lifesciences Corp., Abbott, Medtronics, Corcym, Labcor, Valtech Cardio Ltd., Sorin S.p.A., CroiValve, FOLDAX, Cardia Implant LLC, LivaNova PLC, Cyberonics Inc., Boston Scientific Corporation, Micro Interventional Devices Inc., 4Tech Inc., among others.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In April 2023, Corcym, announced the acquisition of the BioStable Science & Engineering assets including the HAART Aortic Annuloplasty Devices, the world’s only aortic internal annuloplasty devices designed for use during valve repair for aortic insufficiency
- In August 2022, Edwards Lifesciences Corporation, announced the company's PASCAL Precision transcatheter valve repair system received a CE Mark for the treatment of mitral and tricuspid regurgitation (MR and TR)
- In February 2022, CroíValve, a Dublin-based medical device company raised euro8M Series A funding to fund a feasibility clinical study with its DUO Coaptation Valve system
The tricuspid valve repair market is a dynamic and evolving field. The market is expected to grow due to advancements in transcatheter tricuspid valve repair technologies, growing clinical trials, and aggressive organic and inorganic growth strategies followed by the players.
Tricuspid Valve Repair Market Scope
|
Report Metric |
Details |
|
Market Size Projection |
US$2.5 billion by 2028 |
|
Key Drivers |
|
|
Types of Tricuspid Valve Diseases |
|
|
Regional Market Share |
|
|
Competitive Landscape |
Top Key Players: Edwards Lifesciences Corp., Abbott, Medtronics, Corcym, Labcor, Valtech Cardio Ltd., Sorin S.p.A., CroiValve, FOLDAX, Cardia Implant LLC, LivaNova PLC, Cyberonics Inc., Boston Scientific Corporation, Micro Interventional Devices Inc., 4Tech Inc., among others |
|
Recent Market Developments |
|
Key Strategic Questions Addressed
- What is the market size & forecast for the Global Tricuspid Valve Repair Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Tricuspid Valve Repair Market?
- How has COVID-19 impacted the Global Tricuspid Valve Repair Market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the market?
- What is the investment landscape?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the market?
- What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
-
- Market Dynamics
- Drivers
- Restraints
- Key Market Trends
- Industry Speaks
- Market Dynamics
- COVID-19 Impact on the Tricuspid Valve Repair Market
- Global Tricuspid Valve Repair Market - Size & Forecast (2019-2027), By Indication
- Tricuspid Valve Regurgitation
- Tricuspid Valve Stenosis
- Global Tricuspid Valve Repair Market - Size & Forecast (2019-2027), By End User
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Cardiac Catheterization Laboratories
- Others
- Global Tricuspid Valve Repair Market - Size & Forecast (2019-2027), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Player
- Key Strategies Assessment, By Player (2021-2023)
- New Product & Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Abbott Laboratories
- Edward Lifesciences
- Medtronics plc
- Boston Scientific Corporation
- LivaNova
- Corcym
- Labcor
- CroiValve
- Cardia Implant LLC
- Micro Interventional Devices Inc.
- Valtech Cardio Ltd.
- Cyberonics Inc.
- Other Prominent Players
Research Methodology
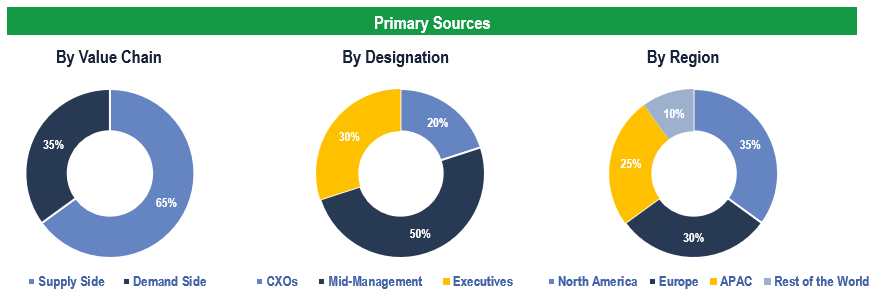
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Ambulatory Surgical Centers (ASCs), Cardiac Catheterization Laboratories, and Other End Users
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.