Point-of-Care Diagnostics Market Size, Global Trends, Industry Share and Regional Analysis for Forecast to 2028
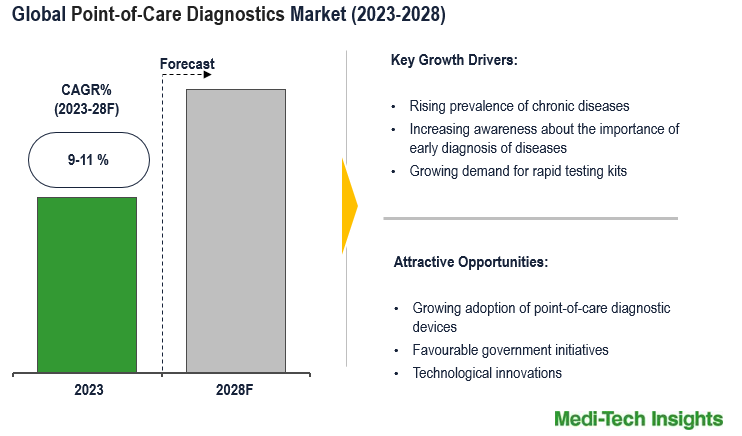
The Global Point-of-Care Diagnostics Market will witness a growth rate of 9-11% by 2028. The market is propelled by the growing prevalence of infectious diseases, the increasing demand for rapid diagnostic kits, ongoing technological advancements, and favourable government support for point-of-care diagnostics devices in key markets. To learn more about the research report, download a sample report.
Point-of-care diagnostics (POC diagnostics) involve medical testing conducted outside traditional laboratories, typically at or near the patient's care location. Aimed at providing swift and convenient results, these tests enable healthcare professionals to make immediate clinical decisions. Key features include rapid results delivery, accessibility in various healthcare settings, user-friendly procedures requiring minimal training, and the ability to prompt immediate clinical actions. With applications spanning infectious diseases, cardiovascular markers, pregnancy, and diabetes monitoring, POC diagnostics contribute to diagnosing, monitoring, and managing diverse health conditions. Portable devices facilitate testing in different locations, particularly in resource-limited areas, and the decentralized nature of POC diagnostics enhances access to testing services. Examples include rapid strep tests, pregnancy tests, and blood glucose monitoring. As technology evolves, new POC diagnostic tools emerge, fostering efficient and patient-centered healthcare practices to improve testing speed and accessibility for enhanced patient care and outcomes.
Beyond the Lab: Accelerating Diagnosis with Point-of-Care Testing for Chronic and Contagious Diseases
The escalating need for swift and accurate diagnosis of chronic and contagious diseases has spurred a growing demand for advanced point-of-care testing. The demand for these innovative testing devices is particularly poised to surge, driven by the increasing prevalence of infectious illnesses. Conditions such as HIV, hepatitis, influenza, and tuberculosis have become major public health concerns globally. The urgency to diagnose and initiate appropriate treatments for these diseases underscores the critical role of point-of-care testing in healthcare settings. These advanced diagnostics not only enhance the speed and efficiency of disease detection but also contribute significantly to the overall management and control of infectious diseases. This trend reflects a broader paradigm shift towards proactive and targeted healthcare solutions, where timely diagnosis becomes a key factor in preventing the spread of diseases and improving patient outcomes. For instance,
- In June 2023, Sysmex introduced the world's first Point-of-Care Testing System in Europe, capable of detecting antimicrobial susceptibility within 30 minutes, focusing on assessing the presence of bacteria and evaluating the effectiveness of antimicrobials using urine samples from patients suspected of urinary tract infections (UTIs)
To learn more about this report, download the PDF brochure
Next-Generation Diagnostics: Enhancing Point-of-Care Testing Through Technological Breakthroughs
Recent advancements in point-of-care testing (POCT) have been significantly shaped by breakthroughs in various technologies, including the incorporation of multiplexing capabilities in diagnostic procedures. This innovation allows for the simultaneous detection and analysis of multiple biomarkers or parameters at the patient's bedside, revolutionizing diagnostic efficiency. Additionally, innovations in cellphone-based technologies, such as the utilization of smartphones, have played a pivotal role in driving cost-effective mobile healthcare and personalized medicine, offering convenient and accessible solutions for users and facilitating the integration of diagnostic capabilities into everyday devices. These collective advancements contribute to the evolution of next-generation diagnostics, enhancing both accessibility and efficiency in healthcare practices. For instance,
- In November 2022, LumiraDx Limited disclosed the ongoing commercial expansion of its HbA1c test, intended for professional use across diverse care settings, where it functions alongside the LumiraDx Platform to monitor HbA1c levels in known diabetic patients and aids in screening and identifying individuals at risk of developing diabetes
Additionally, the development of paper-based assays (PBAs) and lab-on-a-chip (LOC) platforms has ushered in a new era of robust, automated, and simplified point-of-care testing. PBAs, known for their cost-effectiveness, and LOC platforms, designed for miniaturized and integrated diagnostics, contribute to the efficiency and accessibility of diagnostic processes. Novel assay formats further increase the adaptability of point-of-care testing, catering to a wide range of diagnostic needs. One critical aspect that has been addressed in the advancement of these technologies is the stability of reagent storage. Strategies have been devised to enable long-term storage of reagents at ambient temperatures, ensuring the practicality and ease of use of point-of-care testing devices in various settings. The continuous refinement of these technologies holds the potential to revolutionize diagnostic capabilities, offering more accessible and efficient healthcare solutions globally.
Point-of-Care Diagnostics Market: Key Constraints/Challenges
The point-of-care diagnostics market faces challenges related to regulatory compliance, emphasizing the need for rigorous testing and validation due to the diverse nature of devices. Ensuring accuracy and reliability is crucial, and integrating these devices seamlessly into diverse healthcare systems poses additional hurdles. The affordability of production is a significant obstacle, particularly in resource-limited settings, making the successful advancement and integration of point-of-care diagnostics contingent on overcoming these multifaceted challenges.
Regional Dynamics in the Point-of-Care Diagnostics Market
North America has emerged as the focal point of the POC diagnostics market, primarily attributed to its advantageous government policies, widespread awareness of self-testing, and high prevalence of lifestyle diseases. However, the Asia Pacific region is anticipated to witness the swiftest growth in the point-of-care market. This surge is propelled by a burgeoning population, a notable increase in laboratories and diagnostic centers, rising healthcare expenditures, and the growing influence of local manufacturers producing diagnostic kits and reagents.
Point-of-Care Diagnostics Market: Competitive Landscape
Some of the key players operating in the market include Abbott Laboratories, Siemens Healthcare, F. Hoffman-La Roche Ltd, BD (Becton, Dickinson and Company), Trinity Biotech, Sysmex Corporation, Nova Biomedical, Quidel Corporation and Danaher Corporation, among others.
Get a Sample Report for Competitive Landscape Analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In January 2023, Trinity Biotech revealed a strategic 5-year partnership with imaware, designating Trinity Biotech as the lab testing partner for imaware™ and aiming to process over 650,000 test kits annually at its advanced New York-based reference lab facility by the third year, with a focus on delivering integrated diagnostic testing solutions and seamless follow-up confirmatory testing for imaware users
- In January 2021, Thermo Fisher Scientific Inc. revealed its definitive agreement to acquire Mesa Biotech, Inc., a molecular diagnostic company, thereby expanding its portfolio with Mesa's innovative rapid PCR platform technology designed for the detection of infectious diseases, including SARS-CoV-2, Influenza A, and B and other diseases
The point-of-care diagnostics market is expected to gain further momentum in the coming years due to the rising need for quick and reliable diagnostic results, growing awareness of self-testing, increasing prevalence of lifestyle diseases and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
- What is the market size & forecast for the Global Point-of-Care Diagnostics Market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Point-of-Care Diagnostics Market?
- How has COVID-19 impacted the Global Point-of-Care Diagnostics Market?
- What are the major growth drivers, restraints/challenges impacting the market?
- What are the opportunities prevailing in the market?
- What is the investment landscape?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the market?
- What are the key strategies adopted by players?
Find Our Related Reports
Point-of-Care (POC) Blood Diagnostics Market
Diagnostic Imaging Market
Serological Testing Market
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Dynamics
- Global Point-of-Care Diagnostics Market - Size & Forecast (2021-2028), By Test Type
- Covid-19 Testing
- Infectious Disease Testing
- Glucose Testing
- Others
- Global Point-of-Care Diagnostics Market - Size & Forecast (2021-2028), By Product
- Strips and Cards
- Handheld POC Monitoring Devices
- Other Products
- Global Point-of-Care Diagnostics Market - Size & Forecast (2021-2028), By Sample
- Saliva
- Blood
- Urine
- Others
- Global Point-of-Care Diagnostics Market - Size & Forecast (2021-2028), By End User
- Hospitals
- Clinics
- Ambulatory Service Centers
- Diagnostics Centers
- Other End Users
- Global Point-of-Care Diagnostics Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Siemens Healthineers
- Hoffman-La Roche Ltd
- BD (Becton, Dickinson and Company)
- Trinity Biotech, Sysmex Corporation
- Nova Biomedical
- Quidel Corporation
- Danaher Corporation
- Other Players
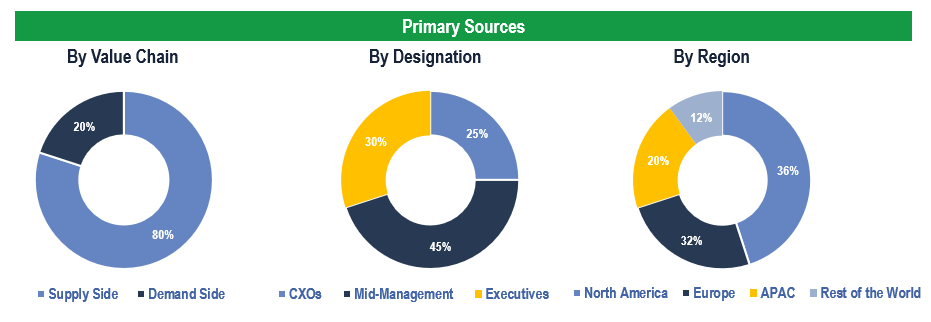
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Hospitals, Clinics, Ambulatory Service Centers and Diagnostics Centers, among others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.