Bioburden Testing Market Size, Share, Industry Analysis, Growth and Forecast to 2024 to 2029
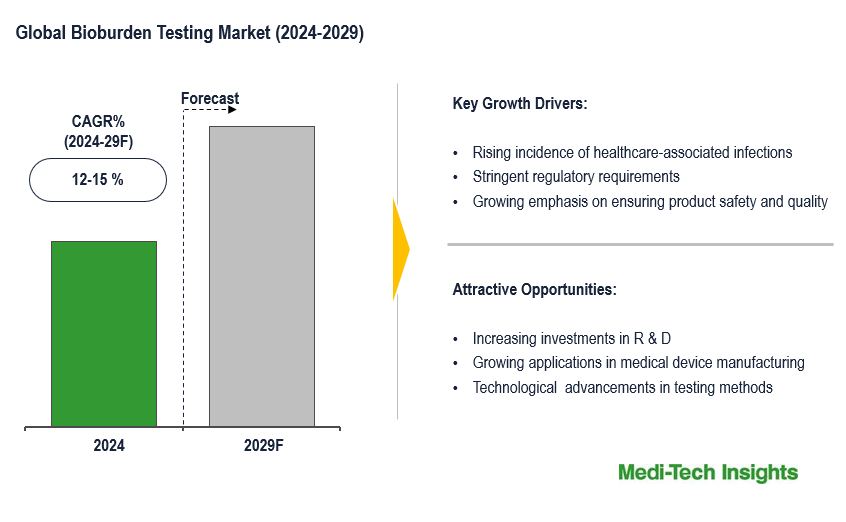
The Global Bioburden Testing Market has been growing steadily at a CAGR of 12-15% due to increasing regulatory requirements and the rising focus on product safety. The market size is influenced by factors such as advancements in testing technologies, stringent regulations, and the expansion of pharmaceutical and biotechnology industries. To learn more about the research report, download a sample report.
Bioburden testing is a crucial aspect of pharmaceutical and medical device manufacturing. It involves assessing the microbial contamination level on products, raw materials, or manufacturing environments. This microbial load consists of bacteria, fungi, yeasts, moulds, and other microorganisms. By quantifying the bioburden, companies can evaluate the effectiveness of their sterilization processes and ensure compliance with regulatory standards.
Bioburden testing plays a critical role in maintaining product quality and safety by preventing contamination-related issues that could compromise patient health or product efficacy. The testing typically includes sampling, culturing, and enumeration of microorganisms present. Regulatory bodies often require bioburden testing as part of quality control measures to comply with Good Manufacturing Practices (GMP). This testing helps identify potential sources of contamination and ensures that products meet regulatory standards for microbial limits.
Driving Forces Behind the Growth of the Bioburden Testing Market
The bioburden testing market is witnessing significant growth driven by multiple factors. Stringent regulatory requirements from bodies like the FDA and EMA necessitate comprehensive bioburden testing to ensure product safety and compliance with quality standards, thereby increasing awareness among industry stakeholders. Moreover, the escalating prevalence of infectious diseases and the growing demand for sterilized medical devices are driving market expansion. The rising focus on quality assurance and patient safety further fuels the demand for bioburden testing services and products. As healthcare facilities prioritize the prevention of healthcare-associated infections and the maintenance of sterile environments, the importance of effective bioburden testing is underscored, propelling the market growth. For instance,
- In August 2022, Lonza introduced the Nebula® Multimode Reader, the inaugural reader qualified for Lonza's turbidimetric, chromogenic, and recombinant endotoxin detection methods, enabling direct comparison of absorbance-based and fluorescence-based endotoxin assays and streamlining maintenance and validation through WinKQCL® Software, thereby enhancing data integrity compliance and facilitating efficiency in endotoxin testing programs as part of Lonza's Complete Testing Solutions
- In June 2022, Berkshire Sterile Manufacturing unveiled its sterility isolator, enabling onsite sterility testing for GMP batches, a service anticipated to expedite release times for the majority of its clients
To learn more about this report, download the PDF brochure
Innovative Solutions in Bioburden Testing: Harnessing Technology for Accuracy and Efficiency
The bioburden testing market is undergoing a significant transformation fueled by advanced technologies such as rapid microbial detection methods like ATP bioluminescence and PCR-based techniques offering reduced testing times with better sensitivity for accurate microbial contamination assessment. These innovations, coupled with automation and robotics, enhance testing efficiency and accuracy while reducing human errors. Automated systems enable high-throughput testing, improving efficiency, reproducibility, and labour cost reduction. The adoption of at-line and in-process bioburden monitoring technologies addresses the increasing complexity of biologics and cell therapy production, ensuring sterility and contamination prevention throughout manufacturing without the need for external lab testing. For instance,
- In the early second quarter of 2023, Merck Millipore's life science division introduced the Milliflex Rapid System 2.0, a solution tailored for the pharmaceutical sector, offering swift bioburden and sterility testing capabilities, adaptable for both standalone and networked operations
Furthermore, the integration of AI and IoT in bioburden testing optimizes efficiency and accuracy by enabling real-time monitoring and automation. These technologies allow for continuous monitoring of environmental parameters and automated sample handling, reducing costs and turnaround times. Platforms like the Sartorius Digital Bioburden Testing Platform exemplify this integration, offering centralized sample management and automated data analytics through AI, revolutionizing workflow and resource utilization in bioburden testing.
Key Constraints
Challenges associated with the bioburden testing market include the complexity of microbial populations, which can vary widely and may require sophisticated testing methods for accurate assessment. Additionally, the need for skilled personnel to perform tests accurately poses a challenge, particularly in regions where access to trained professionals may be limited. High costs associated with implementing advanced testing technologies and maintaining compliance with evolving regulatory standards further add to the challenges faced by industry players. Interpretation of test results and ensuring consistency and reproducibility across different testing facilities also present ongoing challenges. Addressing these challenges will be crucial for sustaining growth and ensuring the effectiveness of bioburden testing in maintaining product quality and safety.
Regional Dynamics and Market Trends in Bioburden Testing
The bioburden testing market is experiencing significant growth globally, with various regions contributing to its expansion. In North America, stringent regulatory standards set by organizations like the FDA, coupled with a well-established pharmaceutical and biotechnology industry, drive market dominance. The region's advanced healthcare infrastructure and increasing awareness of infectious diseases further bolster demand for bioburden testing services. In Europe, a similar trend is observed, with strong regulatory standards and a focus on product quality shaping market dynamics. Investments in healthcare infrastructure and research and development activities further propel market growth in the region.
The Asia Pacific region emerges as a key growth driver, fueled by factors such as outsourcing pharmaceutical manufacturing to countries like China and India. Rising healthcare expenditure and a growing awareness of infection control measures contribute to market expansion. In Brazil, steady growth is witnessed, supported by investments in healthcare infrastructure and a burgeoning pharmaceutical industry. Collaboration between industry players and regulatory agencies aims to standardise testing protocols and enhance product safety. However, economic uncertainties and market access challenges pose potential constraints to growth in the region. Overall, the bioburden testing market is characterized by a shared commitment to ensuring product safety and compliance with regulatory standards across regions, driving continuous expansion and innovation in testing methodologies.
Competitive Landscape
Prominent players in the market include Charles River Laboratories International Inc., Merck KGaA, SGS SA, Nelson Laboratories, LLC, Thermo Fisher Scientific Inc., Becton Dickinson and Company, Wuxi Apptec and bioMérieux SA among others.
Get a sample report for competitive landscape analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner market share. For instance,
- In May 2022, Thermo Fisher and Charles River partnered with robotics company Multiply Labs to advance the automation of cell therapy production, with Thermo Fisher contributing Heracell VIOS incubators aimed at automation, and Charles River focusing on enhancing quality control processes to minimize human error and expedite manufacturing timelines
- In May 2022, Merck announced plans to enhance its membrane and filtration manufacturing capabilities in Ireland, with investments totalling around €440 million to expand capacity in Carrigtwohill and establish a new facility at Blarney Business Park in Cork
The Bioburden Testing Market is expected to gain momentum in the coming years due to stringent regulatory requirements, the rising focus on product safety, the expansion of biopharmaceutical companies, technological advancements, strategic collaborations and aggressive organic and inorganic growth strategies followed by the players.
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Bioburden TestingMarket?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Bioburden Testing Market?
-
How has COVID-19 impacted the Global Bioburden Testing Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Dynamics
- Global Bioburden Testing Market - Size & Forecast (2021-2028), By Product Type
- Consumables
- Culture Media
- Reagents & Kits
- Instruments
- Others
- Global Bioburden Testing Market - Size & Forecast (2021-2028), By Testing Type
- Aerobic Count Testing
- Anaerobic Count Testing
- Fungi/Mould Count Testing
- Spore Count Testing
- Global Bioburden Testing Market - Size & Forecast (2021-2028), By Application Type
- Raw Material Testing
- Medical Device Testing
- In-Process Material Testing
- Sterilization Validation Testing
- Equipment Cleaning Validation
- Global Bioburden Testing Market - Size & Forecast (2021-2028), By End User
- Pharmaceutical and Biomedical Companies
- Medical Device Manufacturers
- Contract Manufacturing Organizations
- Others
- Global Bioburden Testing Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Players
- Key Strategies Assessment, By Player (2021-2023)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Other Developments
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Charles River Laboratories International Inc.
- Merck KGaA
- SGS SA
- Nelson Laboratories, LLC
- Thermo Fisher Scientific Inc.
- Wuxi Apptec
- bioMérieux SA
- Becton, Dickinson and Company
- Other Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
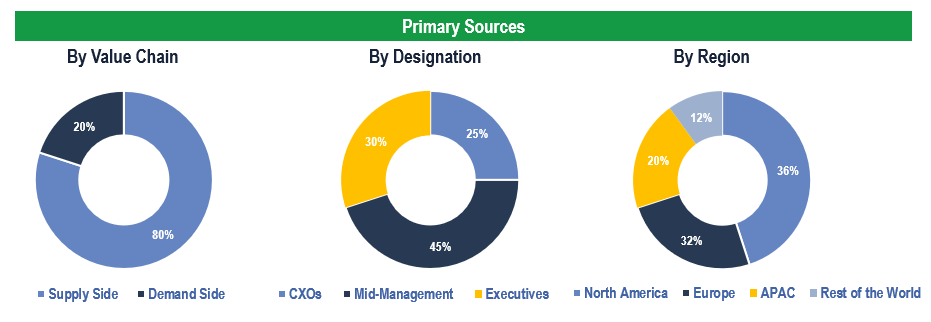
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical & Biotechnology Companies, Medical Device Manufacturers, and Contract Manufacturing Organizations, among others
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.