Bioprocess Containers Market – Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2024 to 2029

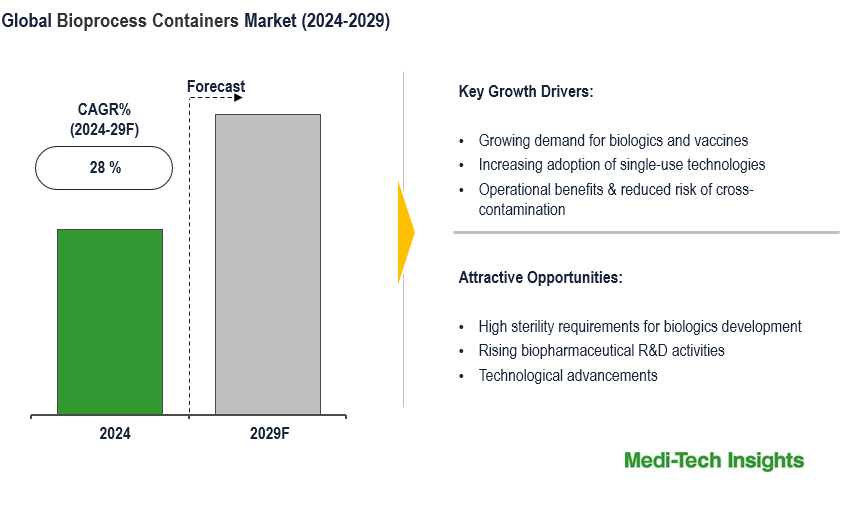
The Global Bioprocess Containers Market is set to witness a healthy growth rate of 28% by 2029. Major advantages of ready-to-use single-use bioprocess container systems, growing demand for biologics & vaccines, and increasing biopharmaceutical R&D activities are some of the key factors driving the market growth. To learn more about the research report, download a sample report.
Bioprocess containers are specialized vessels used in the biopharmaceutical industry to cultivate, store, and transport biological materials and solutions. They are designed to maintain sterility and integrity, safely handling sensitive biological substances such as cells, proteins, and other bioproducts. These containers come in various forms, including bags, tanks, and bottles, and are often made from materials that resist contamination and degradation. Their applications span from small-scale laboratory research to large-scale commercial production, playing a crucial role in the development and manufacturing of vaccines, monoclonal antibodies, and other therapeutic agents.
Advantages of Ready-To-Use Single-Use Bioprocess Container Systems Fuels Its Demand
Over the years, there has been an increase in the adoption of single-use bioprocess containers (BPCs) because of their demonstrated performance, and cost- and time-saving benefits. Single-use technology (SUT) products are widely accepted for manufacturing vaccines and biologics. Some of the key benefits that are driving its demand are:
- Operational Benefits & Reduced Risk of Cross-Contamination:Bioprocess container systems reduce the risk of cross-contamination. Moreover, the elimination of cleaning-in-place and sterilization-in-place (CIP/SIP) systems reduces setup, maintenance, and validation times, thereby enabling increased output
- Ready Integration:Bioprocess container components work well in all stages of the production of therapeutic biologics and vaccines
- Reduction in Cost of Goods Sold (COGS):Bioprocess container systems reduce capital investment and labor costs, which in turn leads to a reduction in COGS
- Scalability:Bioprocess container systems are well suited for use from benchtop to production scale processes
To learn more about this report, download the PDF brochure
High Development & Manufacturing Costs of Biologics Coupled with Its Patent Expiry Likely to Drive the Demand for Single-Use Bioprocessing Technologies
Biologics currently hold nearly half of the pharmacological market for oncology. The Center of Biosimilars stated that by 2023, the expiration of patents on almost 20 oncology biologics could increase the availability of biosimilars in cancer care, reduce costs, and consequently boost the use of single-use systems in biosimilar development, thereby raising the demand for bioprocess containers.
The development of biologics including cell & gene therapies and therapeutic vaccines demand high sterility and therefore biologics are increasingly being manufactured using single-use equipment and consumables. As the development and manufacturing costs of biologics are very high, companies operating in the market are increasingly focusing on techniques that are more cost-effective than traditional bio-manufacturing methods. In an endeavour to manufacture cost-effective products, several biopharmaceutical manufacturers are incorporating single-use bioprocessing technologies into their development processes.
“Biopharmaceutical manufacturers use a range of bioprocess containers (BPCs) during the production and storage of biopharmaceuticals. The trend of “single-use” or “disposable” BPC technologies in the biopharmaceutical industry enables greater flexibility and better use of production facilities that are increasingly designed for multiple products.”- Senior Director, Leading Bioprocess Container (BPC) Manufacturer, United States
Risk of Leachables from Single-use Bioprocess Containers – A Deterrent for Bioprocess Containers Market Growth
Single-use assembly products are made of processed plastic materials and they frequently face the risk of contamination from the container due to leachables.
Leachables are chemical compounds that migrate into the drug formulation from any product contact material (such as elastomeric, plastic, glass, stainless steel, or coating components) as a result of direct contact with the drug formulation under normal process conditions or accelerated storage conditions and are found in the final drug product.
Leachables from single-use bioprocess containers (BPCs) are a source of process-related impurities that have the potential to alter the product quality of biotherapeutics and affect patient health. However, despite these challenges, single-use technologies uptake is expected to grow as suppliers & end-users are working together on this issue. Proper risk assessments, characterization of extractable profiles according to more standardized protocols, toxicological assessments, leachable studies, and matured guidance guidelines are some of the mitigation steps undertaken by stakeholders to confront the problem of leachables.
Innovative Trends and Future Directions in Bioprocess Containers
Several innovative bioprocess container-related products have been launched, incorporating new features like film technology and advanced sensors to enhance the efficiency, reliability, and performance of biopharmaceutical manufacturing, thereby driving market growth. The industry is trending towards customization and modularization in bioprocess container design, driven by manufacturers' need for flexible, adaptable solutions for different production processes. This trend aims to reduce costs and improve efficiency while maintaining product quality and purity. Future developments will include new bioprocess containers and single-use components like pumps and filters. With sustainability becoming increasingly important, these single-use containers will play a crucial role in reducing the need for cleaning and sterilization processes in the biopharmaceutical sector. For instance,
- In April 2023, Merck launched its Ultimus Single-Use Process Container Film for Mobius 3D process containers, offering 10 times greater abrasion resistance than other single-use bioprocessing films, thereby minimizing product loss, supporting healthy cell growth, and improving operational efficiency
- In September 2022, Entegris, Inc. introduced its F/T (Freeze & Thaw) Optimizer at the BioProcess International Conference in Boston, an interactive customer tool using proprietary algorithms to virtually test up to eight equipment and process combinations, providing life sciences customers with unparalleled insights and cost-effective cold chain solutions without physical testing
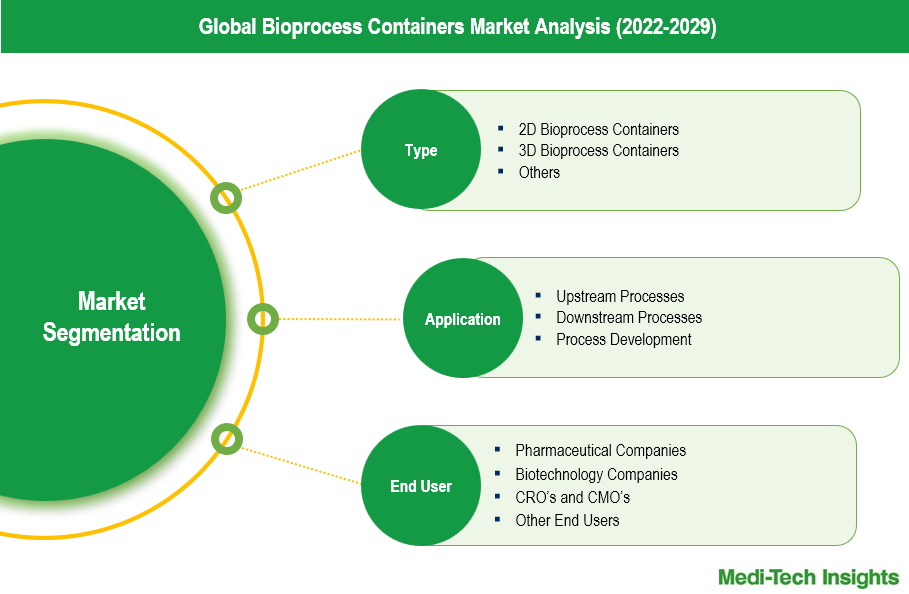
Overview of Bioprocess Container Types
Bioprocess containers are a critical component in the biopharmaceutical industry, available in various types including 2D bioprocess containers, 3D bioprocess containers, and other specialized formats. The 2D bioprocess containers, often used for smaller volumes and simpler applications, represent a significant segment of the market. The 3D bioprocess containers, designed for larger volumes and more complex applications, hold a slightly larger market share due to their efficiency in large-scale bioprocessing operations. The remaining portion of the market is comprised of other types of bioprocess containers, including speciality containers and customized solutions, catering to niche applications and specific customer requirements. The diversity in container types ensures that the varied needs of biopharmaceutical production processes are met, supporting the industry's growth and innovation.
To learn more about this report, download the PDF brochure
Application Segments of Bioprocess Containers
Bioprocess containers are utilized across various stages of biopharmaceutical production, including upstream processes, downstream processes, and process development. In upstream processes, bioprocess containers are essential for cell culture and fermentation, playing a vital role in the initial stages of biomanufacturing. Downstream processes, which involve purification and separation, also heavily rely on these containers for their effectiveness in handling and storing biological products. Additionally, in process development, bioprocess containers are used extensively to optimize and scale up production processes, ensuring consistency and efficiency. Each of these application segments contributes significantly to the overall market, highlighting the versatile and indispensable nature of bioprocess containers in the biopharmaceutical industry.
Competitive Landscape Analysis: Bioprocess Containers Market
The Bioprocess Containers market is marked by the presence of key players such as Sartorius Stedim Biotech, Thermo Fisher Scientific, Danaher Corporation, Merck Millipore, Meissner Corporation, Cole-Parmer Instrument Co, Optimum Processing Inc, Avantor Inc, and Entegris, Inc among others.
Get a sample report for competitive landscape analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, acquisitions, and new product launches to garner market share. For instance,
- In April 2022, Thermo Fisher Scientific opened a new 55,000-square-foot facility in Ogden, Utah, to manufacture highly customizable bioprocess container systems, which are essential for the delivery, processing, separation, storage, and transportation of critical liquids in the development of vaccines and breakthrough therapies
- In July 2021, Cytiva and Pall Corporation announced a $1.5 billion investment over two years to expand manufacturing capacity for life sciences products at 13 sites, addressing the growing demand for biotechnology solutions
- In June 2021, Avantor, Inc. announced its acquisition of RIM Bio, a China-based manufacturer specializing in single-use bioprocess bags and assemblies for biopharmaceutical manufacturing applications
The market is expected to gain further momentum in the coming years due to technological advancements, rising R&D investments, new product launches, and aggressive organic and inorganic growth strategies followed by the players.
Bioprocess Containers Market Futures and Scope
| Report Scope | Details |
| Base Year Considered | 2023 |
| Historical Data | 2022 - 2023 |
| Forecast Period | 2024 - 2029 |
| CAGR (2024-2029) | 28% |
| Segment Scope | Product, Application, End User |
| Regional Scope |
|
| Key Companies Mapped | Sartorius Stedim Biotech, Thermo Fisher Scientific, Danaher Corporation, Merck Millipore, Meissner Corporation, Cole-Parmer Instrument Co, Optimum Processing Inc., Avantor Inc., and Entegris, Inc. among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Bioprocess Containers Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Bioprocess Containers Market?
-
How has COVID-19 impacted the Global Bioprocess Containers Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
-
- Market Dynamics
- Drivers
- Restraints
- Key Market Trends
- Industry Speaks
- Market Dynamics
- Key Revenue Pockets
- Global Bioprocess Containers Market - Size & Forecast (2021-2028), By Type
- 2D Bioprocess Containers
- 3D Bioprocess Containers
- Others
- Global Bioprocess Containers Market - Size & Forecast (2021-2028), By Application
- Upstream Processes
- Downstream Processes
- Process Development
- Global Bioprocess Containers Market - Size & Forecast (2021-2028), By End User Type
- Pharmaceutical Companies
- Biotechnology Companies
- CRO’s and CMO's
- Other End Users
- Global Bioprocess Containers Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Player
- Key Strategies Assessment, By Player (2021-2023)
- New Product & Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Sartorius Stedim Biotech
- Thermo Fisher Scientific
- Danaher Corporation
- Merck Millipore
- Meissner Corporation
- Cole-Parmer Instrument Co
- Optimum Processing Inc
- Avantor Inc
- Entegris Inc
- Other Prominent Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical & Biopharmaceutical Companies, CROs & CMOs, and Academic & Research Institutes and Other End Users
Breakdown of Primary Interviews
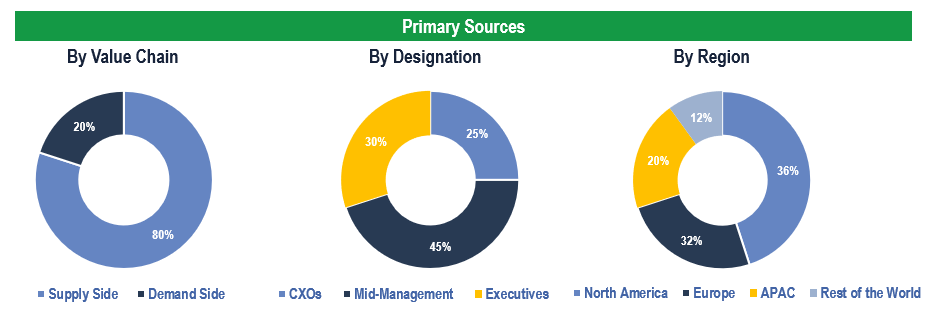
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.