Global Contract Research Organization (CRO) Services Market Size & Trends Report Segmented by Service Type (Clinical Research, Early-phase Development, Consulting), Therapy Area (Oncology, Vaccines), End User (Pharmaceutical & Biotechnology Companies) & Regional Forecast to 2030
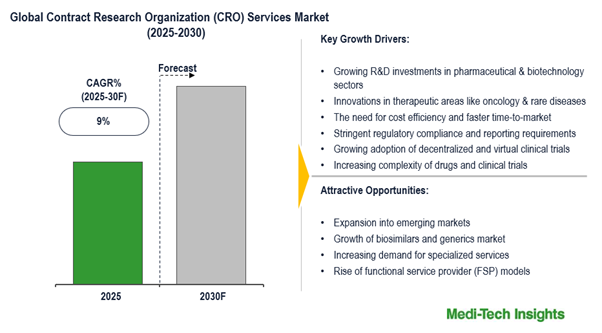
The global contract research organization (CRO) services market is expected to witness a growth rate of 9% in the next five years. Growing R&D investments in pharmaceutical and biotechnology sectors; innovations in therapeutic areas like oncology, and rare diseases; the need for cost-efficiency & faster time-to-market; stringent regulatory compliance/reporting, growing adoption of decentralized and virtual clinical trials, and increasing complexity of drugs and clinical trials are some of the key factors driving the CRO services market growth. To learn more about the research report, download a sample report.
Report Overview
A contract research organization (CRO) provides comprehensive clinical trial services for the pharmaceutical, biotechnology, and medical device sectors. CRO services majorly include clinical research services, early-phase development services, laboratory services, consulting services, among others. Some of the typical reasons why the pharmaceutical, biotechnology, and medical devices industries outsource to CROs include, better return on R&D investments, lack of internal capabilities (esp. small biopharma companies), increasing complexity in developing targeted therapeutic areas like immuno-oncology therapies, stringent regulatory requirements, to focus on core competencies, strategic choice, and time and cost-efficiency among others.
To learn more about this report, download the PDF brochure
Growing Investments from Governments, Private Companies, and Venture Capitalists to Drive Market Growth
In recent years, fundings and investments in CRISPR technology have been significant, showcasing the growing interest and support for this innovative field. In February 2024, CRISPR Therapeutics secured approximately USD 280 million through an agreement to sell its common shares to a select group of institutional investors. This investment aims to accelerate their gene-editing programs and expand their therapeutic pipeline. Another notable example is the Indian biotechnology start-up CrisprBits, which raised USD 250,000 in pre-seed funding from US-based VJ Group in February 2023. This funding is intended for developing CRISPR-based diagnostics to detect pathogens and antimicrobial resistance genes. Likewise, in July 2023, Pfizer Inc. made a USD 25 million equity investment in Caribou Biosciences, Inc. a leading clinical-stage CRISPR genome-editing biopharmaceutical company. These examples highlight the substantial financial support and confidence in the potential of CRISPR technology.
To learn more about this report, download the PDF brochure
Market trend towards full-service CROs/one-stop-shop model
The shift toward full-service CROs is driven by the complexities of modern drug development. Biopharma companies prefer CROs that offer end-to-end services, from pre-clinical research to commercialization, ensuring streamlined processes, cost efficiency, and reduced timelines. Full-service CROs provide scalable resources, specialized expertise, and global reach, enabling seamless international trials and regulatory compliance. Their ability to manage entire projects eliminates the need for multiple vendors, improving efficiency. With advanced technologies and therapeutic expertise, these CROs deliver high-quality outcomes, making them the preferred choice for navigating the evolving drug development landscape and accelerating market entry.
Competitive Landscape Analysis
The global contract research organization (CRO) services market is marked by the presence of established and emerging market players such as IQVIA, Labcorp, Syneos Health, Thermo Fisher Scientific, Parexel, ICON, Charles River, WUXI Apptec, Pharmacon Beijing, and SGS; among others. Some of the key strategies adopted by market players include new service launches, strategic partnerships and collaborations, and geographic expansion.
Report Scope
| Report Scope | Details |
| Base Year Considered | 2024 |
| Historical Data | 2023 – 2024 |
| Forecast Period | 2025 – 2030 |
| Growth Rate | 9% |
| Market Drivers |
|
| Attractive Opportunities |
|
| Segment Scope | Service Type, Therapy Area, End User |
| Regional Scope |
|
| Key Companies Mapped | IQVIA, Labcorp, Syneos Health, Thermo Fisher Scientific, Parexel, ICON, Charles River, WUXI Apptec, Pharmacon Beijing, and SGS among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Market Segmentation
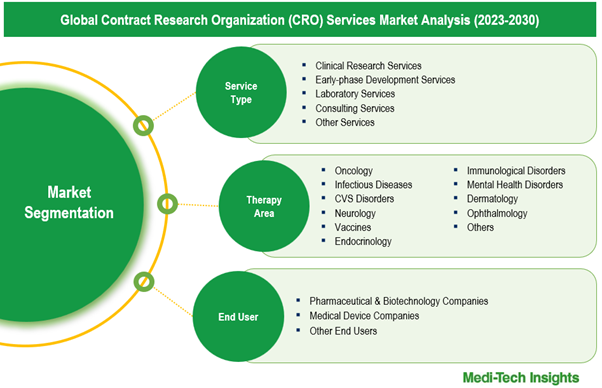
This report by Medi-Tech Insights provides the size of the global contract research organization (CRO) services market at the regional- and country-level from 2023 to 2030. The report further segments the market based on service type, therapy area, end user.
- Market Size & Forecast (2023-2030), By Service Type, USD Million
- Clinical Research Services
- By Phase
- Phase III
- Phase II
- Phase I
- Phase IV
- By Study Design
- Interventional
- Real World Evidence (RWE)
- Early-phase Development Services
- Chemistry, Manufacturing and Controls Services
- Preclinical Services
- Pharmacokinetics/ Pharmacodynamics Services
- Toxicology Testing Services
- Other Preclinical Services
- Discovery Studies
- Laboratory Services
- Analytical Testing Services
- Physical Characterization Services
- Raw Material Testing Services
- Batch Release Testing Services
- Stability Testing Services
- Other Analytical Testing Services
- Bioanalytical Testing Services
- Analytical Testing Services
- Consulting Services
- Other Services
- By Phase
- Clinical Research Services
- Market Size & Forecast (2023-2030), By Therapy Area, USD Million
- Oncology
- Infectious Diseases
- CVS Disorders
- Neurology
- Vaccines
- Endocrinology
- Immunological Disorders
- Mental Health Disorders
- Dermatology
- Ophthalmology
- Others
- Market Size & Forecast (2023-2030), By End User, USD Million
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Other End Users
- Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
- North America
Key Strategic Questions Addressed
- What is the market size & forecast of the CRISPR technology market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the CRISPR technology market?
- What are the key trends defining the market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players?
- Introduction
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2025-2026)
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- Contract Research Organization (CRO) Services Market Snapshot (2025-2030)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Growing R&D investments in pharmaceutical and biotechnology sectors
- Innovations in therapeutic areas like oncology and rare diseases
- The need for cost efficiency and faster time-to-market
- Stringent regulatory compliance and reporting requirements
- Growing adoption of decentralized and virtual clinical trials
- Increasing complexity of drugs and clinical trials
- Restraints
- Regulatory and ethical challenges
- Intellectual property (IP) and data security concerns
- Limitations related to adequate patient recruitment and retention for clinical trials
- Changing complexities associated with clinical trial
- Opportunities
- Expansion into emerging markets
- Growth of biosimilars and generics market
- Increasing demand for specialized services
- Rise of functional service provider (FSP) models
- Drivers
- Key Market Trends
- Growing emphasis on real-world evidence (RWE) and patient-centric trials
- Integration of omics technologies
- Unmet Market Needs
- Industry Speaks
- Market Dynamics
- Global Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Service Type, USD Million
- Introduction
- Clinical Research Services
- By Phase
- Phase III
- Phase II
- Phase I
- Phase IV
- By Study Design
- Interventional
- Real World Evidence (RWE)
- Early-phase Development Services
- Chemistry, Manufacturing and Controls Services
- Preclinical Services
- Pharmacokinetics/ Pharmacodynamics Services
- Toxicology Testing Services
- Other Preclinical Services
- Discovery Studies
- Laboratory Services
- Analytical Testing Services
- Physical Characterization Services
- Raw Material Testing Services
- Batch Release Testing Services
- Stability Testing Services
- Other Analytical Testing Services
- Bioanalytical Testing Services
- Consulting Services
- Other Services
- Analytical Testing Services
- By Phase
- Global Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Therapy Area, USD Million
- Introduction
- Oncology
- Infectious Diseases
- CVS Disorders
- Neurology
- Vaccines
- Endocrinology
- Immunological Disorders
- Mental Health Disorders
- Dermatology
- Ophthalmology
- Others
- Global Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By End User, USD Million
- Introduction
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Other End Users
- Global Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Region, USD Million
- Introduction
- North America Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Country, USD Million
- US
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Canada
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- US
- Europe Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Country, USD Million
- UK
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Germany
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- France
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Italy
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Spain
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Rest of Europe
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- UK
- Asia Pacific (APAC) Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), By Country, USD Million
- China
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Japan
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- India
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Rest of Asia Pacific
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- China
- Latin America (LATAM) Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Middle East & Africa (MEA) Contract Research Organization (CRO) Services Market Size & Forecast (2023-2030), USD Million
- Market Size & Forecast, By Service Type (USD Million)
- Market Size & Forecast, By Therapy Area (USD Million)
- Market Size & Forecast, By End User (USD Million)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Key Player Comparison
- Segment-wise Player Mapping
- Market Share Analysis (2024)
- Company Categorization Matrix
- Dominants/Leaders
- New Entrants
- Emerging Players
- Innovative Players
- Key Strategies Assessment, By Player (2022-2025)
- New Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Company Profiles* (Business Overview, Financial Performance**, Products Offered, Recent Developments)
- IQVIA
- Labcorp
- Syneos Health
- Thermo Fisher Scientific
- Parexel
- ICON
- Charles River
- WUXI Apptec
- Pharmacon Beijing
- SGS
- Other Prominent Players
Note: *Indicative list
**For listed companies
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
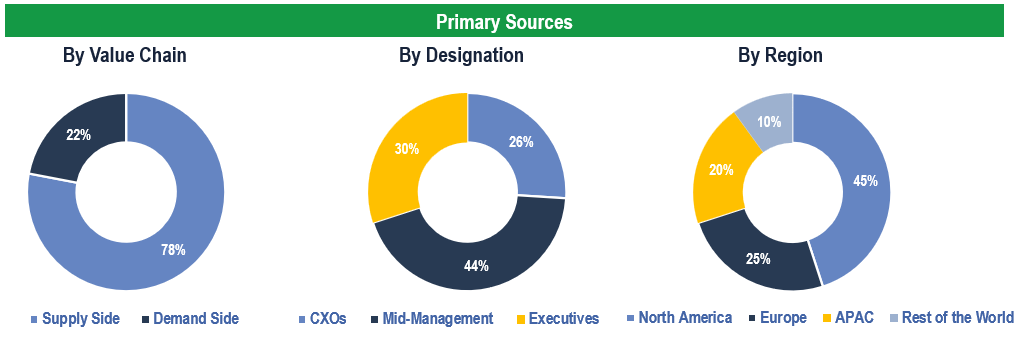
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical and Biotechnology Companies, Academic and Government Research Institutes, Contract Research Organizations (CROs) and Other End Users
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.