Digital PCR (dPCR) and Real-Time PCR (qPCR) Market is Revolutionizing Molecular Diagnostics: with 7% Growth

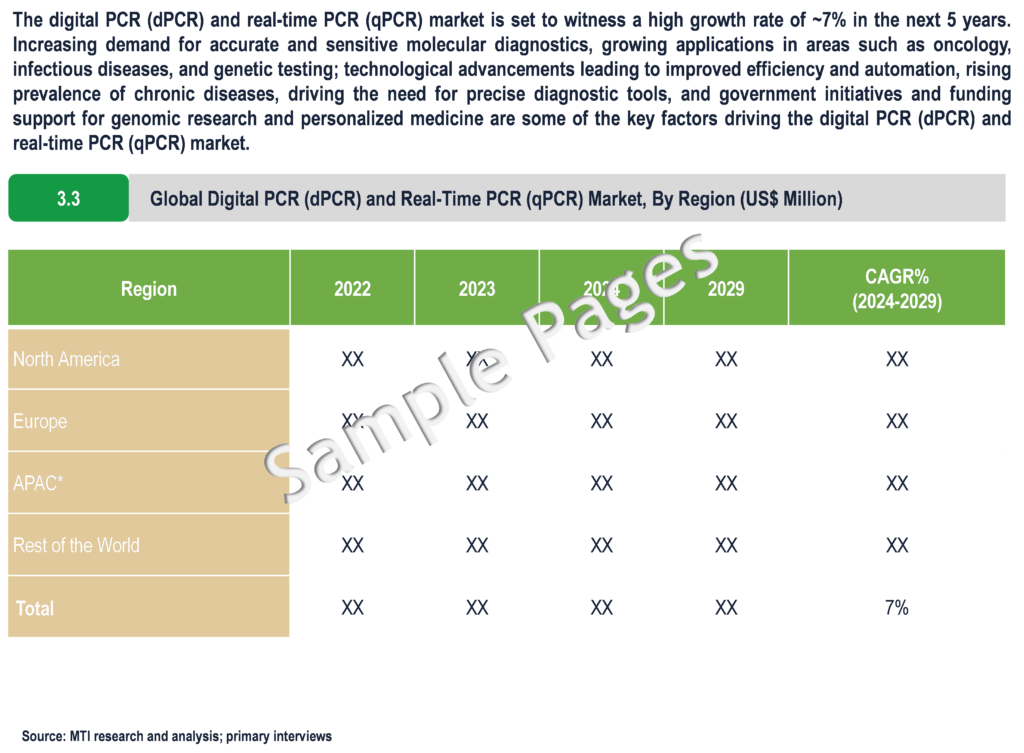
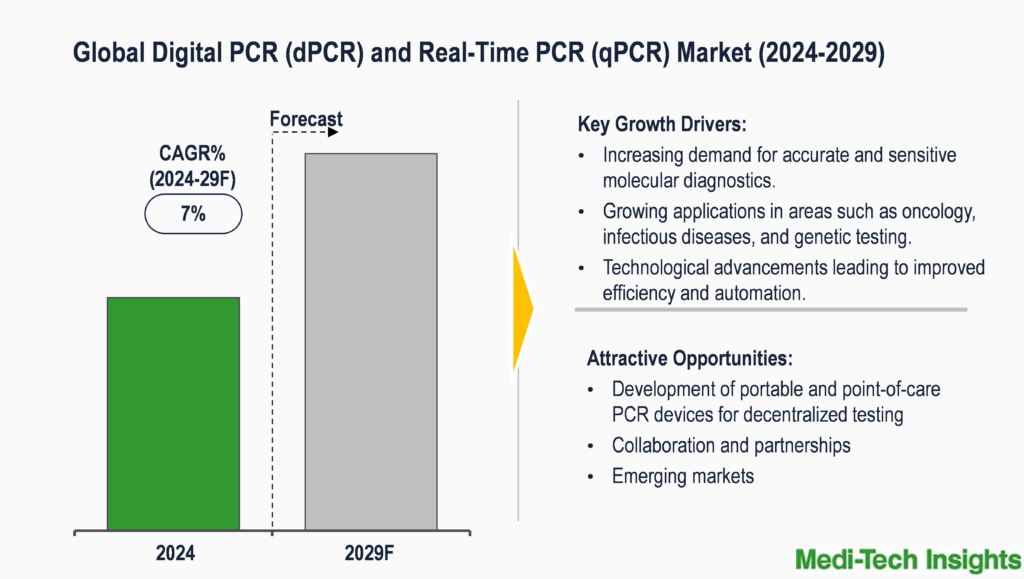
The digital PCR (dPCR) and real-time PCR (qPCR) market is set to witness a high growth rate of ~7% in the next 5 years. Increasing demand for accurate and sensitive molecular diagnostics, growing applications in areas such as oncology, infectious diseases, and genetic testing; technological advancements leading to improved efficiency and automation, rising prevalence of chronic diseases, driving the need for precise diagnostic tools, and government initiatives and funding support for genomic research and personalized medicine are some of the key factors driving the digital PCR (dPCR) and real-time PCR (qPCR) market.
The market is marked by the presence of key players such as Thermo Fisher Scientific, Inc. (US); Danaher Corporation (US); F. Hoffmann-La Roche Ltd (Switzerland); Qiagen (Germany); Bio-Rad Laboratories, Inc. (US); Agilent Technologies, Inc. (US); Becton, Dickinson and Company (US) and others. To learn more about the research report, download a sample report.
Digital PCR (dPCR) and Real-Time PCR, also referred to as quantitative PCR (qPCR), are advanced techniques in molecular biology for the precise detection and quantification of nucleic acids. Both techniques offer highly sensitive and specific detection capabilities. Digital PCR is the third generation of PCR that enables absolute quantification by dividing a sample of DNA, cDNA, or RNA into many individual microreactions. Some microreactions contain one or more molecules, while others contain none. Each microreaction undergoes PCR amplification and analysis independently. Positive microreactions, containing amplified product, are distinguished from negative ones. By counting the number of positive reactions, the initial copy number and density of target DNA can be calculated using Poisson statistics. Real-time PCR allows monitoring of the PCR progress in actual time. qPCR quantifies products with standard curves and provides low tolerance to interfering substances. It measures the fraction of negative microreactions to determine absolute copies, unlike traditional PCR that collects results after the reaction is complete. In addition to improved accuracy, sensitivity, and rapidity, one of the principal advantages of the real-time PCR over basic PCR is that this technique provides a reliable quantification relationship between the number of starting target sequences (before the amplification by PCR) and the amount of amplicon accumulated in a particular PCR cycle.
Growing applications of dPCR and qPCR in oncology, infectious diseases, and genetic testing to propel market demand
The applications of Digital PCR (dPCR) and Real-Time PCR (qPCR) are rapidly expanding across various fields, including oncology, infectious diseases, and genetic testing. In oncology, these advanced PCR techniques are pivotal for detecting rare mutations in cancer biopsy samples, quantifying copy number variations (CNV), and identifying genetic markers associated with cancer diagnosis. For instance, dPCR is instrumental in pinpointing minor DNA targets like point mutations and chromosomal translocations, aiding in precise cancer diagnostics and monitoring of microbial resistance. In oncology, these advanced PCR techniques play a crucial role in the detection and monitoring of cancer biomarkers, aiding in early diagnosis, prognosis, and treatment decision-making. For instance, studies have shown that Real-Time PCR assays targeting specific genetic mutations, such as BRAF V600E in melanoma or epidermal growth factor receptor (EGFR) mutations in lung cancer, have significantly improved patient outcomes by guiding targeted therapies (Source: NCBI). This enables earlier diagnosis and targeted therapies.
In infectious diseases, dPCR and qPCR play a crucial role in quantifying viral loads, monitoring microbial pathogens, and conducting liquid biopsies for less invasive disease monitoring through the detection of circulating tumour DNA (ctDNA) in blood samples. dPCR and Real-Time PCR are indispensable tools for the rapid and accurate detection of pathogens, including viruses, bacteria, and parasites. Particularly during the COVID-19 pandemic, Real-Time PCR-based tests have been pivotal in diagnosing infections, monitoring disease spread, and assessing the efficacy of public health interventions. Due to its speed and ability to monitor disease progression in real-time, it plays a crucial role in detecting viruses like HIV (sensitivity exceeding 99%) (Source: NCBI).
Moreover, in genetic testing, both dPCR and qPCR are essential for gene expression analysis, miRNA expression profiling, and GMO detection by providing accurate quantification of gene expression levels and detecting genetically modified organisms in crops for regulatory compliance. These techniques enable precise quantification of gene expression levels, identification of genetic variants, and screening for hereditary diseases. With their sensitivity, specificity, and ability to analyze multiple targets simultaneously, dPCR and Real-Time PCR are poised to continue driving innovation in personalized medicine and precision diagnostics. qPCR is used in non-invasive prenatal testing (NIPT) to identify chromosomal abnormalities in foetuses with high accuracy (>99%) ((Source: NCBI)
The market for these technologies is expected to grow significantly due to factors such as the rising incidence of target infectious diseases, genetic disorders, continuous advancements in PCR technologies, and the increasing importance of PCR in biomarker discovery. The precision, sensitivity, and absolute quantification capabilities of dPCR and qPCR are driving their adoption in diverse applications within oncology, infectious diseases, and genetic testing fields, revolutionizing molecular diagnostics worldwide. As the understanding of genetic factors underlying diseases deepens and the demand for targeted therapies and personalized treatment strategies increases, the applications of dPCR and Real-Time PCR are projected to expand further, driving market growth and innovation in healthcare.
To learn more about this report, download the PDF brochure
Key strategies adopted by market players and laboratories
Digital PCR and real-time PCR are transforming the healthcare landscape by facilitating precision medicine through the integration of patient-specific genetic data. This shift towards personalized treatment strategies holds the potential for enhanced outcomes and decreased healthcare burdens. The combination of dPCR, qPCR, and CRISPR technologies is ushering in the era of genomic medicine, providing customized therapies at the molecular level. These advancements confer a competitive edge to manufacturers, encouraging leading players to continuously invest in the development of new products and expand their global footprint. This strategic focus is geared towards strengthening their positions in this growing market. Some of the recent developments are listed below-
- In In April 2023, Qiagen launched three new kits for use on its QIAcuity systems and a major new software update designed to expand the portfolio of applications for use of digital PCR technology in areas like cell and gene therapies, DNA and RNA quantification, as well as food and pharmaceuticals safety
- In August 2023, Roche launched its digital polymerase chain reaction (PCR) system, the Digital LightCycler. This next-generation system detects disease and is designed to accurately quantify trace amounts of specific DNA and RNA targets not typically detectable by conventional PCR methods
- In February 2023, Thermo Fisher Scientific launched its Applied Biosystems TaqPath PCR kits for infectious diseases such as multi-drug-resistant tuberculosis (MTB MDR), M. tuberculosis complex (MTB), hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV) in India.
- In April 2022, Stilla Technologies entered into a co-marketing agreement with Promega Corporation with a view to offer a complete digital PCR workflow solution. With this partnership, the companies will offer an optimized workflow for a wide range of applications including liquid biopsy, sentinel pathogen testing, infectious disease assays, overall cancer research and drug discovery.
- In September 2021, Thermo Fisher Scientific launched the Applied Biosystems QuantStudio Absolute Q Digital PCR System, the first fully integrated digital PCR (dPCR) system designed to provide highly accurate and consistent results within 90 minutes.
North America expected to hold a major share in the digital PCR (dPCR) and Real-Time PCR market
From a geographical perspective, North America holds a major market share of digital PCR (dPCR) and real-Time PCR (qPCR) market. This can be mainly attributed to the growing emphasis on personalized medicine in the region, presence of multinational diagnostics companies supporting high R&D investments and ongoing research and development efforts in leading to continuous technological advancements in PCR techniques, enhancing their efficiency, sensitivity, and accuracy. Furthermore, the prevalence of chronic diseases such as cancer and infectious diseases in North America is increasing, creating a greater need for accurate diagnostic tools like dPCR and qPCR for early detection and monitoring. Moreover, supportive governmental initiatives and funding for genomic research and precision medicine initiatives in North America create favourable conditions for market expansion and innovation. Collaborative efforts among academic institutions, healthcare providers, and biotechnology companies in the region further promote innovation and accelerate the adoption of dPCR and qPCR technologies.
Irrespective of the challenges such as high cost associated with equipment and reagents, Complexity in data analysis and interpretation, and challenges pertaining to Integration of dPCR and qPCR into existing laboratory workflows, among others, the global dPCR and qPCR market has high potential to grow at a significant rate and is expected to gain further momentum in the coming years due to a rising prevalence of chronic diseases, driving the need for precise diagnostic tools, government initiatives and funding support for genomic research and personalized medicine, and d Technological advancements leading to improved efficiency and automation.
Competitive Landscape
Some of the key players operating in the market include Thermo Fisher Scientific, Inc. (US); Danaher Corporation (US); F. Hoffmann-La Roche Ltd (Switzerland); Qiagen (Germany); Bio-Rad Laboratories, Inc. (US); Agilent Technologies, Inc. (US); Becton, Dickinson and Company (US), among others.
Get a Sample Report for Competitive Landscape Analysis
Key Strategic Questions Addressed
-
What is the market size & forecast of the PCR (dPCR) and Real-Time PCR (qPCR) market?
-
What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the PCR (dPCR) and Real-Time PCR (qPCR) market?
-
What are the key trends defining the market?
-
What are the major factors impacting the market?
-
What are the opportunities prevailing in the market?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-up and Top-down Approach
- Market Forecasting
- Executive Summary
- Market Overview
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Market Trends
- Industry Speaks
- Opportunity Analysis
- Market Dynamics
- Global Digital PCR (dPCR) and Real-Time PCR (qPCR) Market- Size & Forecast (2023-2029), By Product & Services
- dPCR Products and Services
- dPCR Instruments
- Droplet PCR
- Chip-based PCR
- BEAMing Digital PCR
- dPCR Reagents and Consumables
- dPCR Software and Services
- dPCR Instruments
- qPCR Products and Services
- Reagents and consumables
- Instruments
- Software and services
- dPCR Products and Services
- Global Digital PCR (dPCR) and Real-Time PCR (qPCR) Market- Size & Forecast (2023-2029), By Application
- Clinical Application
- Infectious disease testing
- Oncology Testing
- Blood Screening
- Transplant Diagnostics
- Liquid Biopsy Testing
- Other Clinical Applications
- Research Application
- Forensic Application
- Environmental Application
- Other Applications
- Clinical Application
- Global Digital PCR (dPCR) and Real-Time PCR (qPCR) Market- Size & Forecast (2023-2029), By End User
- Hospitals and Diagnostic Laboratories
- Academic and Research Institutes
- Pharmaceutical and Biotechnology Companies
- CROs and CDMOs
- Forensic Laboratories
- Other End Users
- Global Digital PCR (dPCR) and Real-Time PCR (qPCR) Market- Size & Forecast (2023-2029), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Market Share Analysis (2022)
- Segment-wise Player Mapping
- Key Strategies Assessment, By Player (2020-2022)
- New Product Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Thermo Fisher Scientific, Inc.
- Danaher Corporation
- Hoffmann-La Roche Ltd
- Qiagen
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- Abbott Laboratories
- Merck KGaA
- Promega Corporation
- Eppendorf SE
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
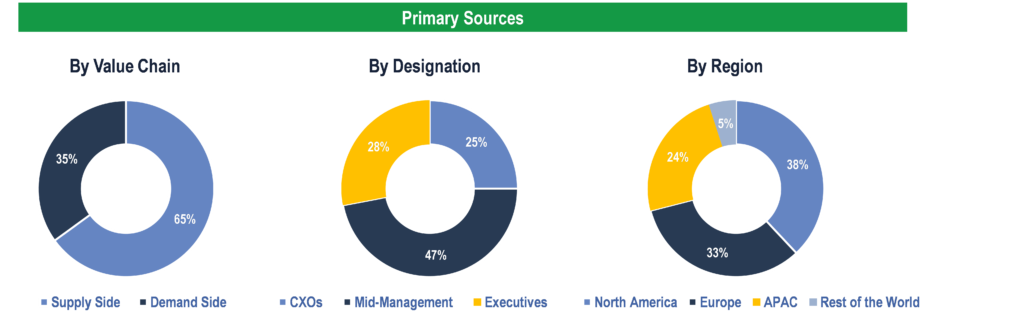
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations (CROs), and Research Institutes & Laboratories, Hospitals and Diagnostic Laboratories, Forensic Laboratories
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down & Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data