Global Biologics CDMO Market Size, Share, Trends, Demand & Growth Analysis and Forecast 2024 to 2029
The Global Biologics CDMO Market is expected to witness a growth rate of 14-16% in the next five years. Growing biopharmaceuticals market, increasing complexity of biologics pushing pharma companies to outsource, rising number of biologics in the pipeline being developed by small and virtual biotechs, burgeoning ecosystem of smaller biopharma companies in China, supported by strong access to funding and favorable regulatory changes are some of the key factors driving the Biologics CDMO market growth. However, the cost of production of regenerative medicines represents a major hurdle on the path to commercialization, reluctance to outsource know-how/IP by pharma and scalability/capacity challenges due to complex nature of biologicsis are some of the factors likely to hinder the market’s growth. To learn more about the research report, download a sample report.
The Biologics CDMO market comprises organizations that provide development and manufacturing services for biologic products. Biologics CDMOs support pharmaceutical and biotechnology companies by offering specialized expertise and advanced facilities required for the production of biologic therapies, including monoclonal antibodies, cell and gene therapies, vaccines, and recombinant proteins.
Growing Biopharmaceuticals Market to Drive Market Growth
The biopharmaceuticals market is rapidly expanding due to the increasing prevalence of chronic diseases, advancements in biotechnology, and the rising demand for personalized medicine. This growth directly impacts the biologics CDMO market. Biologics CDMOs provide specialized services, including drug development, manufacturing, and regulatory support, essential for biopharmaceutical companies aiming to bring complex biologics to market efficiently and cost-effectively. As biopharmaceutical companies face mounting pressure to accelerate development timelines and optimize production, they increasingly outsource to CDMOs for their expertise and advanced technologies. Additionally, the trend towards biologics, such as monoclonal antibodies, cell and gene therapies, and recombinant proteins, necessitates sophisticated manufacturing capabilities that CDMOs offer. Consequently, the expanding biopharmaceuticals market drives the demand for biologics CDMO services, fostering market growth and innovation within the sector.
“As pharmaceutical and biotech companies seek to bring innovative biologics to market, they are increasingly turning to CDMOs to leverage specialized expertise, state-of-the-art technologies, and cost-effective solutions"
- Senior Director, A Global Leading CMO, United States
Increasing complexity of biologics to Fuel Market Growth
Advanced biologics, including cell and gene therapies, require specialized knowledge and cutting-edge technologies for their production. CDMOs offer the necessary expertise and state-of-the-art facilities, enabling faster development and production timelines. By leveraging CDMO capabilities, companies can mitigate risks associated with biologics production, such as scalability and regulatory compliance. Pharma companies can concentrate on their core activities, such as research and development, while CDMOs handle complex manufacturing processes. Also, biopharmaceutical companies often lack the in-house expertise and infrastructure necessary to develop and manufacture these advanced therapies. As a result, they are turning to CDMOs for their specialized knowledge, state-of-the-art facilities, and ability to scale production efficiently. CDMOs offer a range of services, from early-stage development to commercial-scale manufacturing, allowing biopharmaceutical companies to bring complex biologics to market more rapidly and cost-effectively. Moreover, the adoption of advanced technologies such as automation, data-driven manufacturing, and single-use systems by CDMOs enhances their ability to manage the complexities of biologics production. These technologies improve efficiency, reduce human error, and enable rapid adaptation to changing production needs.
As the demand for innovative biologics continues to rise, the role of CDMOs in providing the necessary expertise and infrastructure will become increasingly crucial, driving significant growth in the biologics CDMO market
To learn more about this report, download the PDF brochure
Rising Number of Biologics in the Pipeline are Driving the Biologics CDMO Market
With an increasing focus on innovative therapies, such as monoclonal antibodies, gene therapies, and recombinant proteins, biopharmaceutical companies are advancing a growing number of biologics through various stages of clinical trials. This surge in biologics development requires specialized manufacturing capabilities, which many companies lack in-house. CDMOs provide essential services, including development, manufacturing, and regulatory support, enabling biopharmaceutical companies to efficiently progress their biologics candidates from preclinical stages to commercialization. The complex nature of biologics manufacturing, involving sophisticated technologies and stringent regulatory requirements, further drives companies to seek CDMO partnerships. Additionally, the trend towards personalized medicine and targeted therapies amplifies the need for flexible and scalable manufacturing solutions that CDMOs offer, fueling market growth. This increasing pipeline of biologics thus creates a robust demand for the specialized expertise and infrastructure provided by CDMOs, propelling the market forward:
US Expected to be a Major Growth Engine in Biologics CDMO Market
The US is anticipated to be a major growth engine in the biologics CDMO market due to several key factors. The country's robust biopharmaceutical industry, marked by substantial R&D investments and a high number of biotech firms, drives demand for biologics manufacturing services. Additionally, the US boasts advanced infrastructure and technological capabilities essential for biologics production. Regulatory support, including the FDA's streamlined approval processes, further enhances market growth. The rising prevalence of chronic diseases necessitates innovative biologic therapies, increasing the need for CDMO services. Moreover, strategic partnerships between CDMOs and biopharma companies facilitate efficient production and commercialization of biologics. The US market's overall growth is also fueled by a skilled workforce, extensive clinical research activities, and a strong intellectual property framework, making it a pivotal player in the global biologics CDMO landscape.
To learn more about this report, download the PDF brochure
Product Type Segment Analysis
Monoclonal antibodies (mAbs) command the largest share of the global biologics CDMO market due to their proven efficacy and widespread use in treating various diseases, including cancer, autoimmune disorders, and infectious diseases. Their ability to specifically target and neutralize pathogens or malignant cells has made them a cornerstone in therapeutic regimens. Additionally, advancements in mAb production technologies and increased regulatory approvals have bolstered their market dominance.
On the other hand, the cell and gene therapy CDMO market is expected to witness the fastest growth due to the groundbreaking potential of these therapies in addressing previously untreatable genetic disorders and complex diseases. The increasing number of clinical trials, substantial investment in R&D, and successful commercialization of cell and gene therapies are driving this rapid growth. Furthermore, advancements in gene editing technologies, such as CRISPR, and the growing demand for personalized medicine contribute to the accelerated expansion of the cell and gene therapy CDMO market.
Application Type Scale of Operation Analysis
The Biologics CDMO market can be analyzed based on scale of operation types, such as Pre-clinical & Clinical, and Commercial scale of operation. The commercial scale of operation is the largest and fastest-growing segment in the biologics CDMO market. This segment's dominance is driven by the increasing number of biologics reaching commercial approval and the subsequent need for large-scale production. The surge in demand for biologic drugs, including monoclonal antibodies, vaccines, and cell and gene therapies, necessitates extensive manufacturing capabilities to meet market requirements. Additionally, the commercial segment benefits from long-term manufacturing contracts, ensuring sustained revenue streams for CDMOs. Fast growth in this segment is fueled by technological advancements in biomanufacturing processes, such as single-use technologies and continuous manufacturing, which enhance production efficiency and scalability. The rise in chronic diseases and the global focus on personalized medicine further contribute to the demand for commercial-scale biologics manufacturing. Furthermore, CDMOs are increasingly investing in expanding their commercial production capacities to accommodate the growing pipeline of biologics, solidifying this segment's rapid expansion
Competitive Landscape Analysis: Biologics CDMO Market
The global Biologics CDMO market is marked by the presence of established market players such as Lonza, Catalent, Wuxi Biologics, Samsung Biologics, Fujifilm, Boehringer Ingelheim, Fusion Pharma, Patheon, AGC Bilogics, Eurofins CDMO, and Abbvie., among others.
Get a sample report for competitive landscape analysis
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in the Market
Players operating in this market are adopting both organic and inorganic growth strategies such as expansions, and acquisitions, to garner market share. For instance,
- In July 2024, Agilent Technologies acquired Biovectra for USD 925 million to enhance its CDMO capabilities in biologics, highly potent APIs, and gene editing technologies. This acquisition will expand Agilent's service portfolio and strengthen its presence in the biologics and gene therapy markets
- In March 2022, Lonza agreed to Roche's Genentech Vacaville facility for USD 1.2 billion, significantly enhancing its large-scale biologics manufacturing capacity. The acquisition, set to close in late 2024, will boost Lonza’s production capabilities and create a major West Coast manufacturing hub.
- In January 2024, AGC Biologics expanded its presence in Japan by constructing a new 20,000-square-meter facility in Yokohama, set to open in 2026, to support preclinical through commercial production of various biologics. This USD 350 million investment, backed by Japan’s METI, aims to enhance the country’s vaccine production and strengthen AGC’s global CDMO network
- In May 2023, Aurigene Pharmaceutical Services Limited invested USD 40 million to build a R&D and pilot scale facility in Hyderabad, India for therapeutic proteins, antibodies, and viral vectors. This facility will enhance their capabilities in biologics development, offering integrated services from research to commercial manufacturing, and aims to triple their global reach by 2030
The Biologics CDMO market is expected to gain further momentum in the coming years due to technological advancements, rising R&D investments, new service launches, and aggressive organic and inorganic growth strategies followed by the players.
Future Outlook of the Biologics CDMO Market
The global Biologics CDMO market is expected to gain further momentum in the coming years due to the growing focus on biologics, Innovations in biologic, increasing investment in biopharmaceutical R&D, and advances in manufacturing technologies. These factors collectively contribute to the growth and evolution of the Biologics CDMO market.
Biologics CDMO Market Report Scope
| Report Scope | Details |
| Base Year Considered | 2023 |
| Historical Data | 2022 - 2023 |
| Forecast Period | 2024 - 2029 |
| CAGR (2024-2029) | 14-16% |
| Segment Scope | Product, Application, End User |
| Regional Scope |
|
| Key Companies Mapped | Lonza, Catalent, Wuxi Biologics, Samsung Biologics, Fujifilm, Boehringer Ingelheim, Fusion Pharma, Patheon, AGC Bilogics, Eurofins CDMO, and Abbvie., among others. |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Biologics CDMO Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Biologics CDMO Market?
-
How has COVID-19 impacted the Global Biologics CDMO Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Introduction
- Market Scope
- Market Definition
- Segments Covered
- Regional Segmentation
- Research Timeframe
- Currency Considered
- Study Limitations
- Stakeholders
- List of Abbreviations
- Key Conferences and Events (2023-2024)
- Market Scope
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Bottom-Up Approach
- Top-Down Approach
- Market Forecasting
- Executive Summary
- Biologics CDMO Market Snapshot (2023-2029)
- Segment Overview
- Regional Snapshot
- Competitive Insights
- Market Overview
- Market Dynamics
- Drivers
- Growing biopharmaceuticals market
- Increasing complexity of biologics pushing pharma companies to outsource
- Rising number of biologics in the pipeline being developed by small and virtual biotechs
- Burgeoning ecosystem of smaller biopharma companies in China, supported by strong access to funding and favorable regulatory changes
- Restraints
- The cost of production of regenerative medicines represents a major hurdle on the path to commercialization
- Reluctance to outsource know-how/IP by pharma
- Scalability/capacity challenges due to complex nature of biologics
- Opportunities
- Biosimilars market expansion
- Rapid growth in cell and gene therapies
- Drivers
- Key Industry Trends
- Value Chain Capability Assessment
- Industry SpeaksKey Revenue Pockets
- Market Dynamics
- Global Biologics CDMO Market - Size & Forecast (2021-2028), By Product Type
- Introduction
- Monoclonal Antibodies
- Vaccines
- Recombinant Proteins
- Cell & Gene Therapy
- Others
- Global Biologics CDMO Market - Size & Forecast (2021-2028), By Scale of Operation Type
- Introduction
- Pre-clinical & Clinical
- Commercial
- Global Biologics CDMO Market - Size & Forecast (2021-2028), By Region
- Introduction
- North America Biologics CDMO Market Size & Forecast (2022-2029), By Country, USD billion
- US
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Canada
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Europe Biologics CDMO Market Size & Forecast (2022-2029), By Country, USD billion
- Germany
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- France
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Italy
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- UK
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Spain
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- RoE
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Asia Pacific (APAC) Biologics CDMO Market Size & Forecast (2022-2029), By Country, USD billion
- China
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Japan
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- India
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Rest of Asia Pacific
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Latin America (LATAM) Biologics CDMO Market Size & Forecast (2022-2029), USD billion
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- Middle East & Africa (MEA) Biologics CDMO Market Size & Forecast (2022-2029), USD billion
- Market Size & Forecast, By Product Type (USD billion)
- Market Size & Forecast, By Scale of Operation (USD billion)
- China
- Germany
- US
- Competitive Landscape
- Key Players and their Competitive Positioning
- Market Share Analysis (2023)
- Specialized Vs Full-service CDMO competitive positioning matrix
- Key Strategies Assessment, By Player (2021-2023)
- New Product & Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- Lonza
- Catalent
- Wuxi Biologics
- Samsung Biologics
- Fujifilm
- Boehringer Ingelheim
- Fusion Pharma
- Patheon
- AGC Bilogics
- Eurofins CDMO
- Abbvie
- Other Prominent Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharmaceutical & Biotechnology Companies, and Academic & Research Institutes
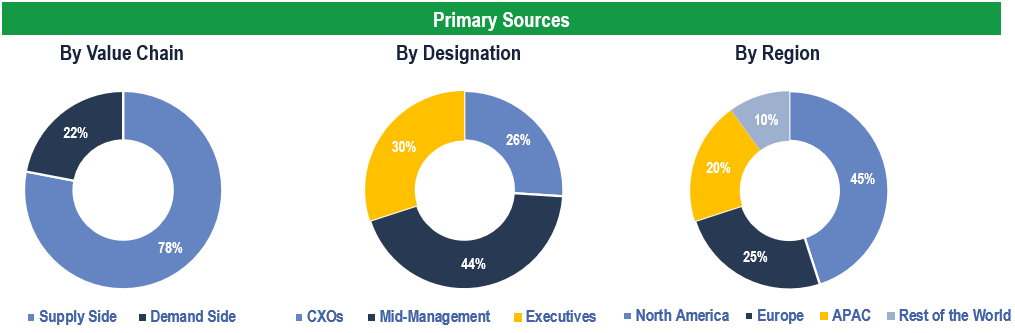
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.