Orphan Drugs CDMO Market Size, Share, Demand, Growth, Analysis & Trends by 2027
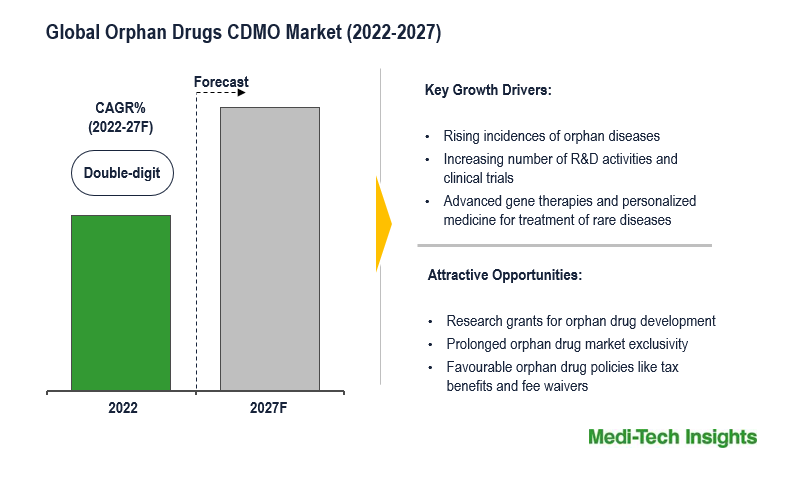
The Global Orphan Drugs CDMO Market is expected to witness a healthy and a double-digit growth rate by 2027. The global market has experienced a healthy and a quick growth due to major factors such as growing focus on rare diseases, and favourable government incentives including tax benefits, fee waivers and longer marketing exclusivity for orphan drugs.
Orphan drugs are designed to address medical conditions impacting a relatively small population, typically fewer than 200,000 individuals in the United States or a similarly low prevalence in other countries. Orphan Drugs CDMOs offer a range of services, including drug formulation, process development, clinical trial materials manufacturing, and commercial-scale production. They work closely with pharmaceutical companies and biotech firms to bring orphan drugs to the market.
Growing Orphan Drugs CDMO Market: Addressing Unmet Medical Needs for Rare Diseases
The Orphan Drugs Contract Development and Manufacturing Organizations (CDMOs) market is poised for a continued growth due to increased investments in therapies for rare diseases. This growth is being fueled by the global orphan drug CDMO market, which is motivated by unmet medical needs and regulatory incentives, making it an appealing sector for CDMOs.
For instance,
- In February 2023, AskBio, which is a subsidiary of Bayer AG, announced today that the European Commission (EC) has granted orphan drug designation to AB-1003, also known as LION-101*. This designation is for the purpose of treating limb-girdle muscular dystrophy (LGMD). AB-1003 represents a groundbreaking investigational gene therapy based on recombinant adeno-associated virus (AAV) and is currently in development as a one-time intravenous (IV) infusion.
In the United States, more than 30 million people are affected by over 7,000 uncommon diseases, many of which are life-threatening and lack effective treatments. Despite the Orphan Drug Act of 1983 and FDA approvals for some rare disease medications, the majority of these conditions still lack approved treatments. The development of drugs, biologics, and medical devices for these rare diseases is challenging due to their complex biology and limited understanding of their natural progression. Given the critical nature of orphan drugs and their often complex manufacturing processes, maintaining high quality and compliance with regulatory standards is paramount. Outsourcing is frequently employed in the manufacturing of orphan drugs to CDMOs which provide quality control systems to meet these requirements. Orphan drugs typically cater to relatively small patient groups, necessitating the creation of smaller batches for clinical and commercial use. Consequently, producing orphan drugs demands flexibility in both batch size and process design, making outsourcing a favored choice.
Key Factors Driving the Rapid Expansion of the Global Orphan Drugs CDMO Market
The expansion of the global orphan drugs CDMO market is heavily shaped by several significant factors that contribute to the growing patient population seeking rare disease treatments. To begin with, there has been a notable increase in the accuracy of rare disease diagnoses due to advancements in medical diagnosis and heightened awareness, which has created a greater demand for orphan drugs. Additionally, governments have implemented legislation that offers pharmaceutical companies various incentives, including extended market exclusivity and tax benefits, thus encouraging them to develop treatments for rare diseases. Furthermore, the global population's consistent growth means that even rare diseases can affect a substantial number of individuals, thereby contributing to the expansion of the market. Moreover, the emergence of precision medicine and genomics has allowed for more precise rare disease diagnosis and treatment, further intensifying the need for customized orphan drugs.
Collaboration between patient advocacy groups, pharmaceutical companies, and research institutions has resulted in increased research funding and enhanced drug development efforts. The globalization of healthcare services has made rare disease treatments more accessible to patients, thus expanding the potential orphan drugs market. Lastly, the designation of orphan drugs status by regulatory agencies serves as an incentive for drug developers, making research into orphan drugs more appealing. In conclusion, the growth of the orphan drugs CDMO market can be attributed to the increasing patient population seeking rare disease treatments, which is facilitated by legislative incentives, medical progress, advocacy initiatives, and the globalization of healthcare services.
For instance,
- In September 2022, Sanofi received FDA clearance for Xenpozyme, an enzyme replacement therapy for acid sphingomyelinase deficiency (ASMD) without central nervous system involvement. Xenpozyme is the FDA-approved drug sanctioned for addressing non-CNS ASMD symptoms in both children and adults in the United States.
Key Constraints/Challenges: Orphan Drugs CDMO Market
The orphan drugs CDMO market has a significant growth potential but it also faces few challenges including limited availability of essential ingredients (API), lengthy formulation development processes, and the necessity for dependable small-scale manufacturing.
North America Accounts for the Largest Share of the Global Orphan Drugs CDMO Market
The global orphan drugs CDMO market is growing at a healthy rate, with a strong presence in North America where many pharmaceutical companies and biotech firms are headquartered. The region's dominance can be attributed to many factors such as rising patient population, favourable regulatory policies and the presence of key market players.
The Asia-Pacific region is anticipated to experience a rapid growth during the forecast period. Rising awareness of rare diseases, increase in the number of clinical trials, and rising adoption of orphan drugs are some of the factors likely to fuel the growth of the orphan drugs CDMO market in the Asia-Pacific region.
Competitive Landscape Analysis: Orphan Drugs CDMO Market
Some of the key players operating in the global orphan drugs CDMO market are Novartis AG, F. Hoffmann-La Roche Ltd, Celgene, Bristol-Myers Squibb Company, Sanofi, Bayer Healthcare, Doppel and LLS Health.
Organic and Inorganic Growth Strategies Adopted by Leading Players to Establish Their Foothold in the Orphan Drugs CDMO Market
All leading players operating in this market are adopting both organic and inorganic growth strategies such as collaborations, and acquisitions to garner a larger market share.
For instance,
- In December 2020, AGC Biologics unveiled its collaboration with Laboratoire Pierre Fabre for the production of ER-004 which is an intra-amniotic drug that is expected to revolutionize the treatment of a rare and incapacitating genetic condition. The role of AGC Biologics is to manufacture GMP material to support the upcoming phase of clinical trials.
The global orphan drugs CDMO market is expected to gain further momentum in the coming years due to the rising number of orphan diseases, favourable government policies like longer marketing exclusivity for orphan drugs, and aggressive organic and inorganic growth strategies followed by the leading market players.
Key Strategic Questions Addressed in this Research Report are as follows:-
- What is the market size & forecast for the global orphan drugs CDMO market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the global orphan drugs CDMO market?
- How has COVID-19 impacted the global orphan drugs CDMO market?
- What are the major growth drivers, restraints/challenges impacting the worldwide orphan drugs CDMO market?
- What are the opportunities prevailing in the orphan drugs CDMO market?
- What is the investment landscape of orphan drugs CDMO market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the orphan drugs CDMO market? What is the competitive positioning of key players?
- Who are the new players entering the global orphan drugs CDMO market?
- What are the key strategies adopted by players operating in orphan drugs CDMO market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2. Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Key Market Trends
3.2. Industry Speaks
4. Global Orphan Drugs CDMO Market - Size & Forecast (2019-2027), By Drug Type
4.1. Biologics
4.2. Non-biologics
5. Global Orphan Drugs CDMO Market - Size & Forecast (2019-2027), By Therapy Type
5.1. Oncology
5.2. Neuromuscular
5.3. Respiratory
5.4. Hematology
5.5. Others
6. Global Orphan Drugs CDMO Market - Size & Forecast (2019-2027), By End User
6.1. Pharmaceutical companies
6.2. Biotechnology companies
6.3. CROs
6.4. Other End Users
7. Global Orphan Drugs CDMO Market - Size & Forecast (2019-2027), By Region
7.1. North America (U.S. & Canada)
7.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
7.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
7.4. Rest of the World (Latin America, Middle East & Africa)
8. Competitive Landscape
8.1. Key Players and their Competitive Positioning
8.1.1. Competitive Positioning of Key Players (2022)
8.1.2. Offerings Assessment, By Player
8.2. Key Strategies Assessment, By Player (2021-2023)
8.2.1. New Service Launches
8.2.2. Partnerships, Agreements, & Collaborations
8.2.3. Mergers & Acquisitions
8.2.4. Geographic Expansion
9. Key Companies Scanned (Indicative List)
9.1. Novartis AG
9.2. F. Hoffmann-La Roche Ltd
9.3. Celgene, Bristol-Myers Squibb Company
9.4. Sanofi
9.5. Bayer Healthcare
9.6. Doppel
9.7. Lubrizol Life Science(LLS) Health
9.8. Other Prominent Players
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
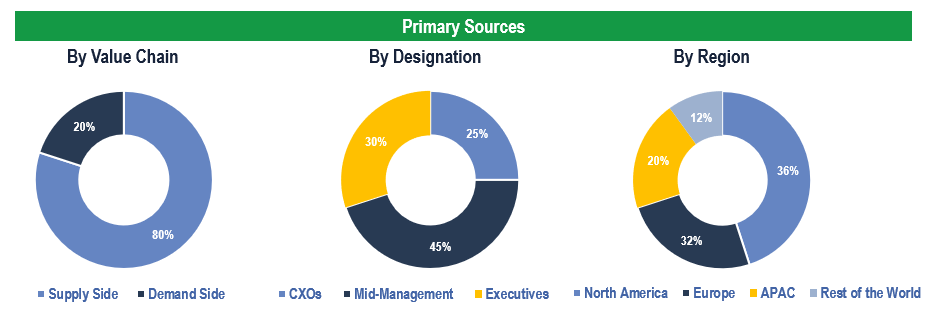
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in CROs, Biotechnology, and Pharmaceutical companies, among others.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.