Pharma Contract Commercialization (CCO) Market Size, Share, Trends, Industry Analysis & Growth Report for Forecast 2024 to 2029

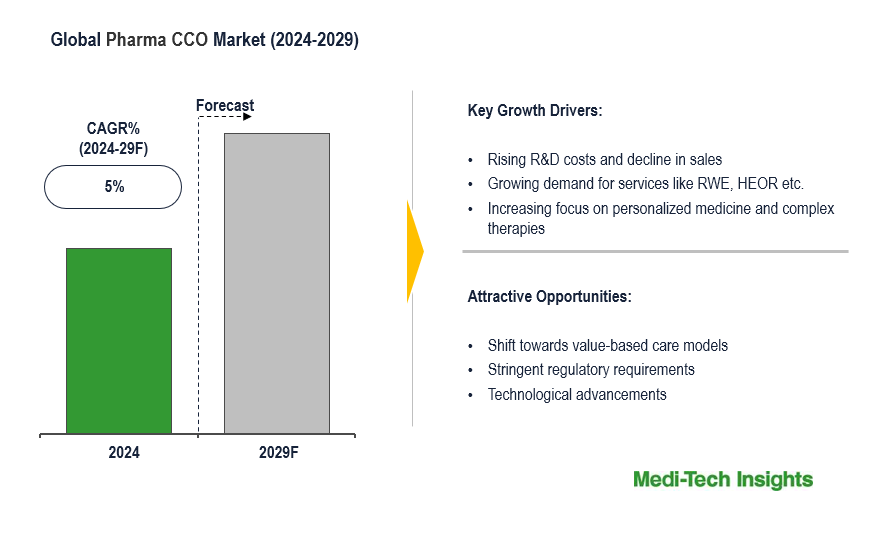
The Global Pharma Contract Commercialization (CCO) Market is expected to witness a CAGR of 5% by 2029. The key factors driving the pharma CCO market growth are growing R&D costs & declining sales of pharmaceutical companies, increasing regulatory scrutiny, growing demand for market access, RWE, HEOR, pricing and reimbursement, regulatory & compliance services, and R&D challenges related to complex therapies. To learn more about the research report, download a sample report.
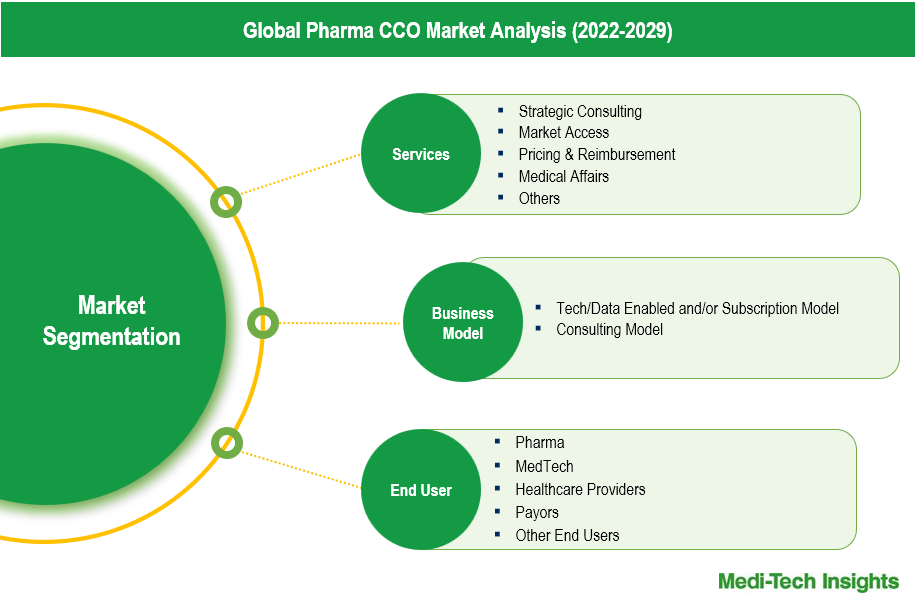
A contract commercial organization (CCO) offers commercial services to Pharma companies to optimize performance, reduce risk, and expedite the delivery of healthcare innovation to patients. Some of the key services offered by CCOs include strategic consulting, market access (including RWE and HEOR), pricing and reimbursement, medical affairs, regulatory and compliance, and other services (data and analytics, and marketing services - HCP engagement, patient engagement, promotional strategies).
Growing demand for Real-World Evidence (RWE) and Health Economics and Outcomes Research (HEOR) drives the Global Pharma CCO market
Real-world data is the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources such as electronic health records (EHRs), medical claims and billing data, observational studies, patient-reported outcomes (PROs), patient-generated health data gathered from various medical devices, among others. Regulatory bodies are increasingly using real-world data (RWD) and real-world evidence (RWE) to monitor and evaluate the post-market safety of approved drugs. RWE complements clinical trial data by providing insights into safety and effectiveness data from a patient’s daily life. It has the potential to impact approvals and accelerate the drug development process. Moreover, payors use real-world data to make coverage decisions, price negotiations and outcomes-based contracting.
HEOR data includes observational data, price comparison, and other types of data that evaluates the economic impact of healthcare interventions, treatment, or conditions. It includes direct medical costs such as medications, hospital days, tests, indirect costs such as unpaid assistance, days lost from work, and decreased productivity, and intangible costs such as pain. It is increasingly being used to generate evidence related to the value of new interventions for reimbursement agencies and local healthcare payers.
“The utilization of RWE and HEOR is expected to expand further in the coming years as it addresses multiple issues such as product safety and risk concerns, cost justification of products, clinical trial designs, among others."-Marketing Director, Pharma CCO Service Provider, U.S.A
To learn more about this report, download the PDF brochure
Consolidation of Clinical Research Organization (CRO) & CCO Operations – A Key Market Trend
Over the years, a number of CROs and CCO companies have achieved synergies by adding commercialization/CRO services to their portfolios through acquisitions, which in turn have allowed them to offer an ‘End-to-End’ solution to their Pharma clients.
For instance:
- In March 2024, Indegene announced its subsidiary, Indegene Ireland, acquired Trilogy Writing & Consulting GmbH, enhancing its specialty medical writing capabilities in clinical, regulatory, safety, and medical content for global market authorization applications
- In February 2024, Real Chemistry announced a significant expansion of its medical education, medical affairs, and HCP communications capabilities by incorporating Avant Healthcare, which offers comprehensive solutions across the entire drug commercialization lifecycle, including medical affairs, promotional education, scientific strategy, and speaker development and management
- In July 2023, Catalyst Clinical Research, a full-service oncology CRO acquired Genpro Research, a next-generation services and technology partner for the pharmaceutical, biotechnology, and medical devices industry with expertise in biometrics, medical writing, RWE, and AI-enabled automation product development.
- In 2017, INC Research Holdings, a leading global Phase I–IV CRO, and inVentiv Health, Inc., a global CRO and CCO announced a merger agreement and in January 2018, INC Research/inVentiv Health changed its brand identity to Syneos Health
To learn more about this report, download the PDF brochure
Comprehensive Segmentation of the Pharma CCO Market: Key Services and Their Impact on Industry Growth
The Pharma CCO market is segmented into key service areas: Strategic Consulting, Market Access, Pricing & Reimbursement, and Medical Affairs, along with other services like data and analytics, and digital marketing. Market Access represents the largest segment due to the critical need for demonstrating product value and securing reimbursement from payers. Strategic Consulting is also significant, providing essential guidance for navigating complex market dynamics. Pricing & Reimbursement services are crucial for optimizing pricing strategies in a globally regulated environment, while Medical Affairs focuses on robust medical communication and education to support product launches and therapeutic education. Other services contribute to the market by leveraging digital tools and analytics to enhance marketing and engagement efforts.
Comprehensive Analysis of End-User Segmentation in the Pharma CCO Market
The end-user segment of the Pharma CCO market is primarily composed of pharmaceutical companies, MedTech firms, healthcare providers, payors, and other end users. Pharmaceutical companies hold the largest market share, leveraging CCO services to enhance market access, regulatory compliance, and commercialization strategies. MedTech firms also significantly rely on CCOs for similar needs, particularly in navigating complex regulatory landscapes and market entry strategies, and they command a substantial share of the market. Healthcare providers utilize these services for improved patient engagement and streamlined operations, representing a notable portion of the market. Payors, focusing on optimizing reimbursement and pricing strategies, account for a significant share as well. Other end users, including biotechnology companies and contract research organizations, benefit from specialized services that support their specific needs in the drug development and commercialization process, constituting a meaningful segment of the market.
Organic and Inorganic Growth Strategies Adopted by the Leading Market Players to Establish Their Foothold in the Global Pharma CCO Market
Leading players operating in the global market are adopting both organic and inorganic growth strategies such as launching new services, acquiring related firms, and entering into collaborations to garner a higher market share.
For instance,
- In March 2024, EVERSANA announced its selection by Tonix Pharmaceuticals Holding Corp. to support the U.S. launch strategy and commercial planning for Tonmya (TNX-102 SL, cyclobenzaprine HCl sublingual tablets), including an assessment of the fibromyalgia landscape and the development of an efficient go-to-market strategy
- In August 2023, Indegene launched Invisage, an AI-enabled hybrid omnichannel sales and marketing platform designed to enhance HCP impact for life sciences companies by optimizing their go-to-market models and personalizing outcomes using data from over 2 million HCPs and 200 million HCP interactions
- In Dec 2022, Red Nucleus, a provider of strategic learning and development, scientific services and advisory, medical communications solutions, and market access and commercialization services exclusively for the life sciences industry acquired AlphaGroup, A scientific, medical affairs, and outcomes communication services company
- In May 2022, Genesis Research, a provider of tech-enabled Real-World Evidence (RWE) and Health Economics and Outcomes Research (HEOR) services, acquired Market Access Transformation (MAT), a leading provider of technology-enabled payer research platforms
- In April 2022, Excelra, a global Data and Analytics provider for Life Science organizations, announced a strategic majority investment in Anlitiks, a HEOR and RWE company
Competitive Landscape Analysis: Pharma CCO Market
Some of the key and established players operating in the global pharma CCO market are EVERSANA, IQVIA, Certara, Indegene, Real Chemistry, Lucid Group, Syneos Health, Genesis Research, Avalere Health, Cheors, Avid Bioservices.
Get a sample report for competitive landscape analysis
The Global Pharma CCO Market is expected to gain further momentum in the upcoming years due to a shift towards value-based care, changing regulatory policies, the need to reduce healthcare burden, improve patient outcomes, reduce costs, and aggressive organic and inorganic growth strategies followed by the key market players.
Pharma Contract Commercialization (CCO) Market Futures and Scope
| Report Scope | Details |
| Base Year Considered | 2023 |
| Historical Data | 2022 - 2023 |
| Forecast Period | 2024 - 2029 |
| CAGR (2024-2029) | 5% |
| Segment Scope | Services, Business Model and End User |
| Regional Scope |
|
| Key Companies Mapped | EVERSANA, IQVIA, Certara, Indegene, Real Chemistry, Lucid Group, Syneos Health, Genesis Research, Avalere Health, Cheors, and Avid Bioservices among others |
| Report Highlights | Market Size & Forecast, Growth Drivers & Restraints, Trends, Competitive Analysis |
Key Strategic Questions Addressed
-
What is the market size & forecast for the Global Pharma CCO Market?
-
What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Global Pharma CCO Market?
-
How has COVID-19 impacted the Global Pharma CCO Market?
-
What are the major growth drivers, restraints/challenges impacting the market?
-
What are the opportunities prevailing in the market?
-
What is the investment landscape?
-
Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
-
Who are the major players operating in the market? What is the competitive positioning of key players?
-
Who are the new players entering the market?
-
What are the key strategies adopted by players?
- Research Methodology
- Secondary Research
- Primary Research
- Market Estimation
- Market Forecasting
- Executive Summary
- Market Overview
-
- Market Dynamics
- Drivers
- Restraints
- Key Market Trends
- Industry Speaks
- Market Dynamics
- Key Revenue Pockets
- Global Pharma CCO Market - Size & Forecast (2021-2028), By Services
- Strategic Consulting
- Market Access (Including RWE and HEOR)
- Pricing & Reimbursement
- Medical Affairs
- Regulatory & Compliance
- Other Services (Data & Analytics and Marketing Services - HCP Engagement, Patient Engagement, Promotional Strategies)
- Global Pharma CCO Market - Size & Forecast (2021-2028), By Business Model
- Tech/Data Enabled and/or Subscription Model
- Consulting Model
- Global Pharma CCO Market - Size & Forecast (2021-2028), By End User Type
- Pharma
- MedTech
- Healthcare Providers
- Payors
- Other End Users
- Global Pharma CCO Market - Size & Forecast (2021-2028), By Region
- North America (U.S. & Canada)
- Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, India, Japan, Rest of Asia Pacific)
- Rest of the World (Latin America, Middle East & Africa)
- Competitive Landscape
- Key Players and their Competitive Positioning
- Competitive Positioning of Key Players (2022)
- Offerings Assessment, By Player
- Key Strategies Assessment, By Player (2021-2023)
- New Product & Service Launches
- Partnerships, Agreements, & Collaborations
- Mergers & Acquisitions
- Geographic Expansion
- Key Players and their Competitive Positioning
- Key Companies Scanned (Indicative List)
- EVERSANA
- IQVIA
- Certara
- Indegene
- Real Chemistry
- Lucid Group
- Syneos Health
- Genesis Research
- Avalere Health
- Cheors
- Avid Bioservices
- Other Prominent Players
The study has been compiled based on extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
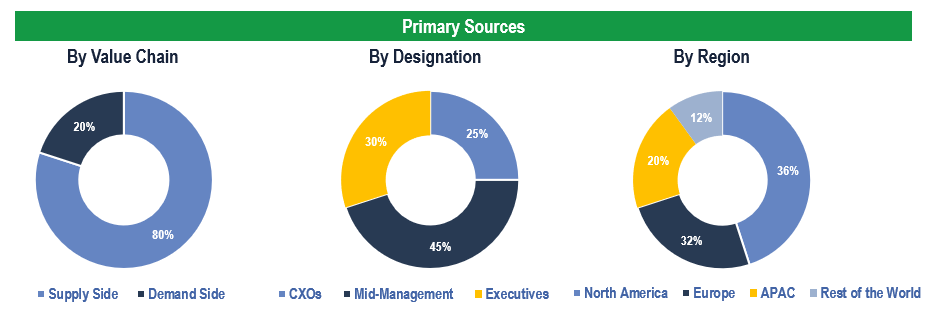
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand-side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Pharma, MedTech, Healthcare Providers, Payors and Other End-users
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.