Cardiac Rhythm Management Devices Market Size, Share, Trends & Applications 2026
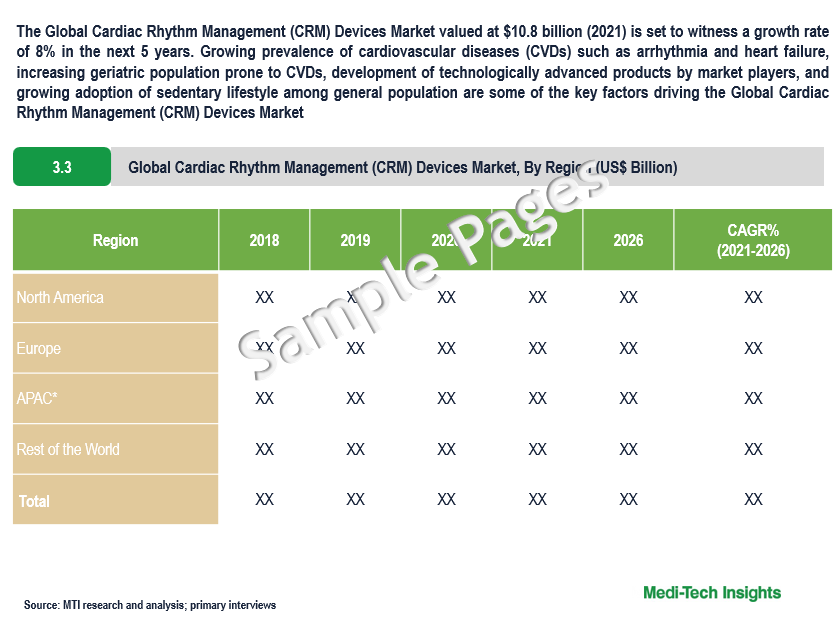
Global Cardiac Rhythm Management Devices Market valued at $10.8 billion (2021), is set to witness a growth rate of 8% in the next 5 years. Growing prevalence of cardiovascular diseases (CVDs) such as arrhythmia and heart failure, increasing geriatric population prone to CVDs, development of technologically advanced products by market players, and growing adoption of sedentary lifestyle among general population are some of the key factors driving the global market growth.
A procedure involving implantation of a device in the upper chest and connected to the heart to control both fast and slow heart rhythms by using electrical pulses is called as cardiac rhythm management (CRM), and the device is called as cardiac rhythm management device. CRM devices perform numerous functions, such as arrhythmias monitoring, cardiac resynchronization for heart failure, bradycardia pacing, defibrillation and anti-tachycardia pacing for tachyarrhythmias.
Technological advancements in CRM devices to fuel its Market Demand
Continuous advances in CRM have enabled to develop efficient devices with increased battery longevity, smaller size, Bluetooth enabled, and MRI-compatible, among others. Such advancements tend to provide competitive edge to manufacturers and therefore, major players are continuously focusing on investing in research activities for new product development and expanding their geographic reach to strengthen their positions in this high growth market. Some of the technological advancements are listed below:
- In May 2022, MicroPort CRM received approval from China’s National Medical Products Administration (NMPA) for its Platinium implantable cardioverter defibrillator (ICD), with an expected lifespan of 13-14 years reducing the risks associated with frequent replacements.
- In April 2022, Abbott’s Aveir single-chamber (VR) leadless pacemaker has received the U.S. Food and Drug Administration (FDA) approval for the treatment of patients with slow heart rhythms in the U.S.
- In January 2022, Medtronic plc received approval from Ministry of Health, Labor and Welfare, Japan to sell and reimburse its Micra AV Transcatheter Pacing System (TPS), one of the world’s smallest pacemakers.
- In July 2020, Abbott received the U.S. Food and Drug Administration (FDA) approval for its Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices that offer MRI compatibility and increased battery longevity.
- In May 2020, Medtronic plc received the U.S. Food and Drug Administration (FDA) approval for its Cobalt and Crome implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy-defibrillators (CRT-D). These devices come with high power and offering connected health technology.
- In March 2019, Biotronik launched 3 Tesla full-body MR conditional ICD and CRT-D devices in the European region, one of the world’s smallest implantable defibrillators with extended battery life and warranty.
Growing prevalence of cardiovascular diseases (CVDs) to boost adoption of Cardiac Rhythm Management Devices Market
Cardiovascular diseases are one of the leading causes of death across the globe. Coronary heart disease, cerebrovascular disease, rheumatic heart disease, and other illnesses are among the group of heart and blood vessel disorders known as CVDs. Heart attacks and strokes account for more than four out of every five deaths caused by CVDs, and one third of these deaths happen before the age of 70. Additionally, behavioral risk factors of heart disease and stroke such as sedentary lifestyle, unhealthy diet, tobacco use, excessive alcohol consumption, and physical inactivity leads to raised blood sugar, increased blood pressure, overweight and obesity among individuals which further contributes to the overall CVD burden.
- According to WHO, 32% of all deaths across the globe i.e., 17.9 million people die due to CVD every year
- As per CDC,
- 659,000 people in the US die from heart disease every year; heart diseases costs $363 billion each year in the US which includes cost of medicines and healthcare services.
- Coronary heart disease alone killed 360,900 people in 2019 in the US and over 18.2 million adults suffering from coronary artery disease live in the US.
- 805,000 people suffer from heart attack in the US every year of which 605,000 are a first heart attack while 200,000 happen to people already had a heart attack.
- According to European Heart Network, CVDs account for over 3.9 million deaths every year in the European region, out of which over 1.8 million deaths occur in the European Union (EU).
- In 2019, the mortality rate of CVDs in China was 364.5 per 100,000 population.
Furthermore, rising geriatric population prone to heart defects due to progressive deterioration in the structure and function of the heart, largely contributes to the high burden of CVDs; thereby triggering the increased adoption of CRM devices.
Issues related to implantable CRM devices – A Deterrent for the Cardiac Rhythm Management Devices Market Growth
Although CRM devices offers multiple advantages to patients and caregivers, there are certain issues related to the use of implantable pacemakers and defibrillators. These include electrical failure, cybersecurity concerns, and high cost of implantable devices.
- Electrical failure: The breakdown of insulation around the wires is the primary cause of electrical failure. Loss of device functionality, inappropriate shocks, and sometimes death are all consequences of lead failures. Implanted defibrillators administer electric shocks to keep hearts beating normally through cables, but these wires are sometimes not dependable and are prone to failure. The CRM devices market has faced a number of products recalled due to electrical failures in their devices. For instance, in December 2020, Boston Scientific recalled its EMBLEM S-ICD due to risk of causing a short-circuit during the delivery of high voltage shocks. Additionally, in August 2019, Abbott recalled its Ellipse ICDs fearing electrical failures caused due to faulty manufacturing process making some of the aluminum wires to be partially exposed.
- Cybersecurity concerns: A cyber attacker who has access to the CRM device could interfere with its radio frequency, alter its memory, and ultimately impair its functionality. According to the US FDA, although the risk of such attacks is low but cannot be considered as zero. Such instances in these devices may impact the adoption rate in near future. For instance, in April 2018, Abbott issued a firmware update, approved by the US FDA, for its 350,000 defibrillators (ICDs and CRT-Ds) due to potential exploitation of cybersecurity vulnerabilities.
Irrespective of the restraints such as electrical failure, cybersecurity concerns, and high cost of these devices; CRM devices market is expected to grow at a significant rate due to rapid advancements in implantable CRM devices. Substantial improvements in terms of increased battery longevity and smaller size along with technological advancements such as Bluetooth enabled and MRI-compatible devices, are expected to further drive the cardiac rhythm management devices market growth.
Competitive Landscape Analysis: Cardiac Rhythm Management Devices Market
The cardiac rhythm management devices market is marked by the presence of key players such as Medtronic (Ireland); Boston Scientific Corporation (US); Abbott Laboratories (US); BIOTRONIK SE & Co. KG (Germany); Stryker Corporation (US); Koninklijke Philips N.V. (Netherlands); and others.
Key Strategic Questions Addressed
- What is the market size & forecast of the Cardiac Rhythm Management (CRM) Devices Market?
- What are historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the Cardiac Rhythm Management (CRM) Devices Market?
- What are the key trends defining the cardiac rhythm management devices market?
- What are the major factors impacting the market?
- What are the opportunities prevailing in the cardiac rhythm management devices market?
- Which region has the highest share in the global market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market?
- What are the key strategies adopted by players operating in cardiac rhythm management devices market?
The study has been compiled based on the extensive primary and secondary research.
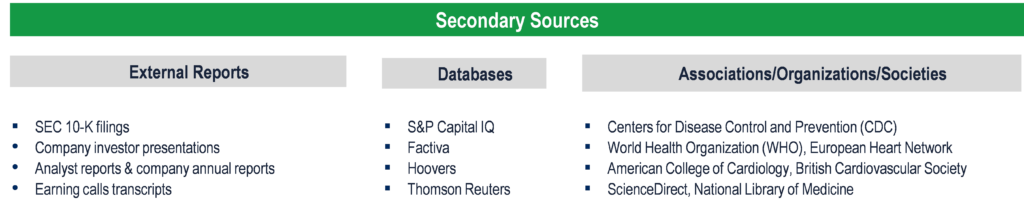
Secondary Research (Indicative List)
Primary Research
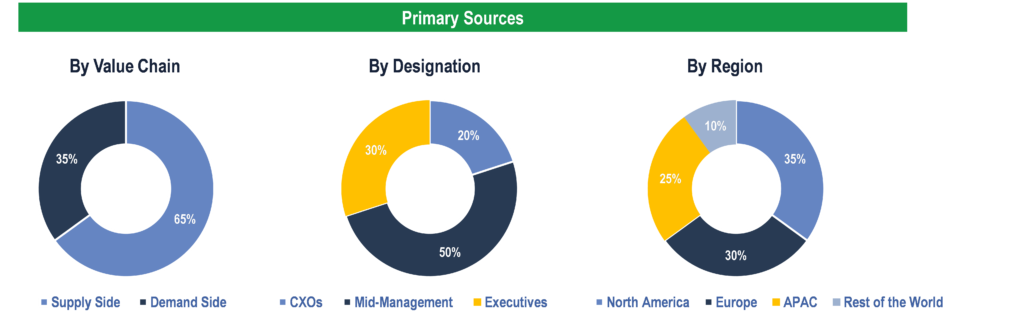
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in Public and Private Hospitals, Cardiac Catheterization Laboratories (Cath Labs), and Clinics.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts.
Data Triangulation
Research findings derived through secondary sources & internal analysis were validated with Primary Interviews, Internal Knowledge Repository, and Company Sales Data.