ENT Devices are used in the identification, therapy, or surgical treatment of any ear, nose and throat disorders. These devices help in correcting problems related to hearing, smell, snoring, or speaking. Some of the ENT devices available in the market are endoscopes, hearing aids, hearing screening instruments, hearing implants, ear tubes, voice prosthesis devices, surgical devices, powered surgical tools, image-guided surgery systems, balloon dilation devices which are used in sinus, CO2 lasers, among others.
Promising Prospects of Artificial Intelligence (AI) in the Global ENT Devices Market
The advent of AI is projected to revolutionize the evolution of image-guided ENT and skull base procedures and favorably impact the diagnosis of laryngeal cancers. Citing the potential prospects of AI in the ENT devices market, all key players are incessantly striving to launch innovative products in the global ENT devices market.
For instance:
- In September 2021, Acclarent launched its first-ever AI-powered ENT technology in the U.S., designed to simplify surgical planning and provide real-time feedback during ENT navigation procedures.
- Similarly, AI is poised to make giant strides in the field of Otolaryngology. The Netherlands based company WSK Medical is optimistic that by the end of the year 2023, European otolaryngologists may be using artificial intelligence (AI) to diagnose laryngeal cancer. The company has developed a platform - Zeno AI - that gives ENT-surgeons a real-time AI analysis of early laryngeal cancer detection that can be utilised during an endoscopic examination (laryngoscopy). Zeno AI considerably reduces time to analyse complex medical data and assists in diagnosing laryngeal cancer faster. The product is not commercialized yet but it is already in use in two European academic medical centers and in proving helpful in identifying laryngeal lesions.
Technological Innovations and Advancements Triggers the ENT Devices Market's Growth
The global ENT devices market has witnessed a significant number of technological advancements and innovations over the years. The technological advancements in ENT devices market is expected to spur its demand in the upcoming years.
For instance:
- In August 2022, Preceptis Medical secured U.S. FDA clearance for expanded indications for use for the Hummingbird® Tympanostomy Tube System (TTS) for office-based pediatric ear tube procedures. The system was previously cleared in children 6-24 months, this new labeling allows in-office procedures in all children six months and older.
- In April 2022, Atmos MedizinTechnik GmbH & Co. KG launched the next generation of endoscopes for ENT and swallowing diagnostics — the Atmos Scope. The flexible HD video nasopharyngolaryngoscope sets new standards with its high image resolution and, via its USB interface, enables mobile use with portable devices.
Fill out the "Quick Inquiry Form" to request a sample copy
Competitive Landscape Analysis: ENT Devices Market
Some of the established players operating in the global ENT devices market are Karl Storz, Medtronic, Stryker, Smith+Nephew, Olympus, Entermed, Acclarent (Part of Johnson & Johnson MedTech), Fujifilm, Aesculap (B Braun), Preceptis Medical, Eakin Surgical, FENTEXmedical GmbH, Sheffmed, PENTAX Medical, Atmos MedizinTechnik GmbH & Co. KG, Collin SAS, Adept Medical, among others.
Organic and Inorganic Growth Strategies Adopted by the Key Market Players to Establish Their Foothold in the Global ENT Devices Market
Leading players operating in this market are adopting organic and inorganic growth strategies such as launching new products, acquiring related firms, and entering into collaborations to garner a higher market share.
For instance,
- In September 2023, Olympus Corporation launched the Vathin E-SteriScope™ single-use flexible video rhinolaryngoscope for use in diagnostic and therapeutic otorhinolaryngological procedures. The E-SteriScope single-use flexible video rhinolaryngoscope is manufactured by Hunan Vathin Medical Instrument and distributed exclusively by Olympus. It is available for sale in the U.S., and its availability in Europe, Middle East and Africa is anticipated later this year.
- In July 2022, Zsquare, a leading developer of high-performance, single-use endoscopes, received Food and Drug Administration 510K clearance to market its first product, the Zsquare ENT-Flex™ Rhinolaryngoscope. It is indicated for use in diagnostic ENT procedures by way of the nose and throat and is the only scalable platform capable of transmitting high-resolution images in flexible, single-use endoscopes.
- In May, 2022, Medtronic completed the acquisition of Intersect ENT, a global ear, nose, and throat (ENT) medical technology leader. The acquisition expands the company's comprehensive ENT portfolio with innovative products used in sinus procedures.
The global ENT devices market is expected to gain a consistent momentum in the upcoming years due to growing number of ear tube procedures (tympanostomy) among kids, rising expectations for sinusitis treatments in outpatient and office settings and aggressive organic and inorganic growth strategies followed by the leading market players.
ENT Devices Market Scope
|
Report Metric
|
Details
|
|
Devices Covered
|
Endoscopes, hearing aids, hearing screening instruments, implants, ear tubes, surgical devices, powered tools, image-guided systems, balloon dilation devices, CO2 lasers, and more.
|
|
AI Impact on the Market
|
Integration of Artificial Intelligence (AI) is revolutionizing image-guided ENT and skull base procedures, enhancing the diagnosis of laryngeal cancers. Key players launching AI-powered technologies for surgical planning and real-time feedback.
|
|
Technological Innovations
|
Significant advancements, e.g., AI integration, expanded indications for devices (e.g., Hummingbird® Tympanostomy Tube System), next-gen endoscopes (e.g., Atmos Scope), and high-resolution flexible video rhinolaryngoscopes.
|
|
Key Players
|
Karl Storz, Medtronic, Stryker, Smith+Nephew, Olympus, Acclarent (Part of Johnson & Johnson), Fujifilm, Preceptis Medical, Atmos MedizinTechnik, and more.
|
|
Recent Developments
|
- Acclarent's AI-powered ENT technology launch (September 2021
- WSK Medical's Zeno AI for laryngeal cancer detection (Projected by end of 2023).
- FDA clearance for expanded indications for Hummingbird® TTS (August 2022).
|
|
Market Drivers
|
Growing ear tube procedures among children, increasing demand for sinusitis treatments in outpatient settings, and aggressive growth strategies by key market players.
|
|
Future Outlook
|
Consistent momentum expected in the global ENT devices market due to various factors, including procedural growth and strategic initiatives by key players.
|
Key Strategic Questions Addressed in this Research Report are as follows:-
- What is the market size and forecast for the ENT devices market?
- What are the historical, present, and forecasted market shares and growth rates of various segments and sub-segments of the ENT devices market?
- What are the major growth drivers, restraints/challenges impacting the ENT devices market?
- What are the opportunities prevailing in the ENT devices market?
- What is the investment landscape of the global ENT devices market?
- Which region has the highest share in the market? Which region is expected to witness the highest growth rate in the next 5 years?
- Who are the major players operating in the market? What is the competitive positioning of key players?
- Who are the new players entering the ENT devices market?
- What are the key strategies adopted by players in global ENT devices market?
1. Research Methodology
1.1. Secondary Research
1.2. Primary Research
1.3. Market Estimation
1.4. Market Forecasting
2. Executive Summary
3. Market Overview
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunities
3.2. Industry Speaks
4. ENT Devices Market - Size & Forecast (2019-2027), By Product
4.1. Diagnostic ENT Devices
4.2. Surgical ENT Devices
4.3. Nasal Splints
4.4. Hearing Implants
4.5. Hearing Aids
5. ENT Devices Market - Size & Forecast (2019-2027), End-user
5.1. ENT Clinics
5.2. Home Use
5.3. Hospitals & Ambulatory Care Settings
6. ENT Devices Market - Size & Forecast (2019-2027), By Region
6.1. North America (U.S. & Canada)
6.2. Europe (UK, Germany, France, Italy, Spain, Rest of Europe)
6.3. Asia Pacific (China, India, Japan, Rest of Asia Pacific)
6.4. Rest of the World (Latin America, Middle East & Africa)
7. Competitive Landscape
7.1. Key Players and their Competitive Positioning
7.1.1. Competitive Positioning of Key Players (2022)
7.1.2. Service Offerings Assessment, By Players
7.2. Key Strategies Assessment, By Player (2021-2023)
7.2.1. New Service Launches
7.2.2. Partnerships, Agreements, & Collaborations
7.2.3. Mergers & Acquisitions
7.2.4. Other Developments
8. Key Companies Scanned (Indicative List)
8.1. Karl Storz
8.2. Medtronic
8.3. Stryker
8.4. Smith+Nephew
8.5. Olympus
8.6. Fujifilm
8.7. Entermed
8.8. Acclarent (Part of Johnson & Johnson MedTech)
8.9. Aesculap (B Braun)
8.10. Atmos MedizinTechnik GmbH & Co. KG
8.11. Eakin Surgical
8.12. FENTEXmedical GmbH
8.13. PENTAX Medical
8.14. Collin SAS
8.15. Sheffmed
8.16. Preceptis Medical
8.17. Adept Medical
8.18. Other Players
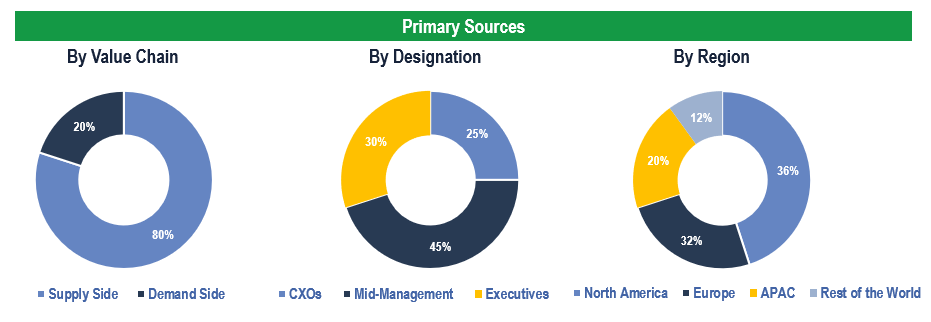
The study has been compiled based on the extensive primary and secondary research.
Secondary Research (Indicative List)
Primary Research
To validate research findings (market size & forecasts, market segmentation, market dynamics, competitive landscape, key industry trends, etc.), extensive primary interviews were conducted with both supply and demand side stakeholders.
Supply Side Stakeholders:
- Senior Management Level: CEOs, Presidents, Vice-Presidents, Directors, Chief Technology Officers, Chief Commercial Officers
- Mid-Management Level: Product Managers, Sales Managers, Brand Managers, R&D Managers, Business Development Managers, Consultants
Demand Side Stakeholders:
- Stakeholders in ENT Clinics, Hospitals, Ambulatory Care Settings, among others.
Breakdown of Primary Interviews
Market Size Estimation
Both ‘Top-Down and Bottom-Up Approaches’ were used to derive market size estimates and forecasts
Data Triangulation
Research findings derived through secondary sources & internal analysis was validated with Primary Interviews, Internal Knowledge Repository and Company’s Sales Data